扩展功能
文章信息
- 郭嘉, 冯杰, 谭贻, 唐传红, 刘艳芳, 周帅, 张劲松
- GUO Jia, FENG Jie, TAN Yi, TANG Chuanhong, LIU Yanfang, ZHOU Shuai, ZHANG Jinsong
- 灵芝液态发酵胞内外多糖的研究进展
- Liquid fermentation of Ganoderma lingzhi for intracellular and extracellular polysaccharides: a review
- 微生物学通报, 2022, 49(10): 4337-4356
- Microbiology China, 2022, 49(10): 4337-4356
- DOI: 10.13344/j.microbiol.china.220184
-
文章历史
- 收稿日期: 2022-02-23
- 接受日期: 2022-04-15
- 网络首发日期: 2022-05-16
2. 上海理工大学健康科学与工程学院, 上海 200093
2. School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China
灵芝(Ganoderma lingzhi),曾用亮盖灵芝(Ganoderma lucidum)当做中国普遍栽培赤芝的拉丁学名,是隶属于真菌界担子菌门多孔菌目灵芝科灵芝属的真菌[1]。始载于《神农本草经》,已被广泛应用了数千年[2-4]。灵芝多糖是其药理活性的主要活性成分,具有抗肿瘤、降血糖、抗炎保肝、调节免疫、保护神经等功效[5-9]。目前,灵芝多糖主要来源于孢子粉、子实体、菌丝体和灵芝发酵液等材料,灵芝材料的获取方式主要包括人工栽培、野外采摘和液态发酵[10]。相较于栽培生产,液态发酵在灵芝优势菌株的诱变、筛选、驯化及基因改良等方面具有周期短、易操作等优势;在探究外源物质对菌株生长及代谢过程影响等方面具有简单、易调控的优势;在调控生长环境及多糖产量等方面具有可控制性强、短时间内可获得大量目标产物、活性物质稳定、易定向富集目标产物及可实现大规模化生产等优势,其研究已得到国内外研究人员的广泛关注[11-15]。本文分别从灵芝多糖的生物合成代谢、培养基组成、发酵工艺和外源添加物对利用灵芝液态发酵法获得胞内外多糖的影响等方面,总结近年来灵芝液态发酵胞内外多糖的研究进展,以期为灵芝液态发酵胞内外多糖的利用及规模化生产提供一定参考和依据。
1 灵芝多糖的生物合成代谢目前,从灵芝中分离出的多糖有200多种,其多糖主链大多是β-葡聚糖,少部分为α-葡聚糖。在β-葡聚糖构成中多为β-(1→3)、β-(1→4)和β-(1→6)葡聚糖,分子量范围为103−106 Da[16-20]。灵芝多糖的单糖组成相对恒定,主要由葡萄糖、甘露糖、半乳糖、果糖、岩藻糖、阿拉伯糖、鼠李糖和木糖等单糖构成[21-22]。灵芝多糖的生物合成代谢是极度复杂又高度协调的系统,有学者通过构建灵芝菌株的基因组规模代谢模型来探究灵芝对碳源的代谢过程及胞外多糖合成途径,也有学者通过同位素示踪技术构建灵芝胞内多糖合成的基本途径,两者在核苷酸糖前体合成途径具有一定相似性[23-26]。研究发现,在灵芝的碳源中心代谢途径中,葡萄糖、半乳糖、甘露糖和果糖在其单糖激酶的作用下,产生相应的磷酸单糖,然后单糖进入三羧酸(tricarboxylic acid,TCA)循环和磷酸戊糖途径(pentose phosphate pathway,PPP),而木糖和阿拉伯糖需先通过氧化还原进入PPP途径形成果糖-6-磷酸;在鼠李糖和岩藻糖代谢过程中,首先分别合成丙酮酸和3-磷酸甘油醛,进而进入循环途径[23]。灵芝多糖的合成大致可以分为3个部分:一是核苷酸糖前体的合成,二是单糖重复
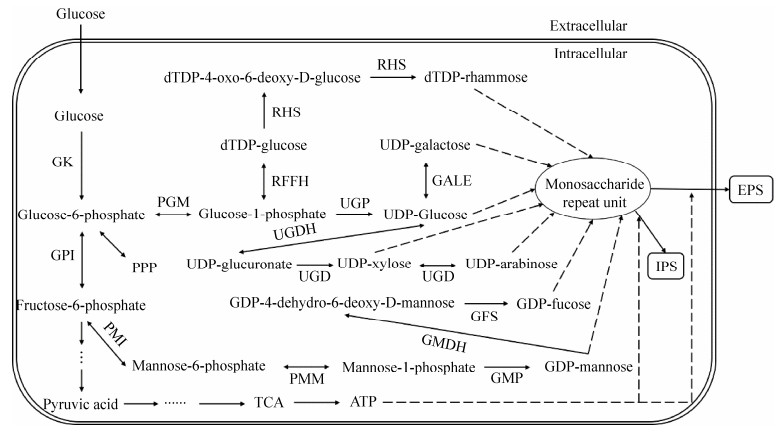
单元在脂质载体上的组装,三是聚合过程[27-29]。在灵芝多糖合成过程中,葡萄糖、阿拉伯糖、木糖、岩藻糖、甘露糖和鼠李糖在单糖激酶和变位酶的催化下生成相应的单糖-1-磷酸,然后在其焦磷酸化酶的作用下合成UDP-葡萄糖、UDP-阿拉伯糖、UDP-木糖、GDP-岩藻糖、GDP-甘露糖和dTDP-鼠李糖等NDP-单糖核苷酸糖前体[23-24]。半乳糖在单糖激酶和异构酶作用下生成葡萄糖-1-磷酸,进而生成核苷酸糖前体;此外,木糖和阿拉伯糖可进入戊糖磷酸途径(PPP)合成果糖-6-磷酸,岩藻糖和鼠李糖通过糖异生途径合成果糖-6-磷酸,果糖在激酶的作用下合成果糖-6-磷酸;果糖-6-磷酸经葡萄糖-6-磷酸异构酶和甘露糖-6-磷酸异构酶作用下生成葡萄糖-6-磷酸和甘露糖-6-磷酸,在变位酶的作用下生成葡萄糖-1-磷酸和甘露糖-1-磷酸,甘露糖-1-磷酸进而可生成GDP-甘露糖和GDP-岩藻糖[23]。然而葡萄糖-1-磷酸除了在焦磷酸化酶作用下形成UDP-葡萄糖外,还可在dTDP-葡萄糖焦磷酸化酶作用下合成dTDP-葡萄糖,进而在鼠李糖合成酶系作用下生成dTDP-鼠李糖,UDP-葡萄糖在UDP-葡萄糖-6-脱氢酶作用下生成UDP-葡萄糖醛酸,进而在UDP-葡萄糖醛酸脱羧酶作用下生成UDP-木糖[25]。在灵芝多糖生物代谢过程中,磷酸葡萄糖变位酶(phosphoglucomutase,PGM)和尿苷二磷酸葡萄糖焦磷酸化酶(UDP-glucose pyrophosphorylase,UGP)均参与核苷酸糖前体的生物合成[30-32]。磷酸葡萄糖变位酶(PGM)是碳代谢中的重要酶,对葡萄糖的磷酸基团转化起着关键作用,其催化葡萄糖-6-磷酸(Glc-6-P)和葡萄糖-1-磷酸(Glc-1-P)之间的可逆转化反应,还存在于糖酵解和D-半乳糖降解的代谢途径之间的分支点[30]。尿苷二磷酸葡萄糖焦磷酸化酶(UGP)催化葡萄糖-1-磷酸和UDP-葡萄糖之间的可逆转化[31]。β-1, 3-葡聚糖合成酶(β-1, 3-glucan synthase,GLS)催化葡萄糖从UDP-葡萄糖重复添加到伸展的糖链[32]。目前,灵芝多糖的核苷酸糖前体合成途径已经清楚,其代谢及合成途径如图 1所示,但单糖重复单元的组装和聚合过程尚不清晰,使得发酵过程中目标产物多糖的糖苷键、支化程度、分子量等较难进行定向调控。
|
| 图 1 灵芝多糖合成代谢的基本途径[23-25] Figure 1 The basic pathway of synthesis and metabolism of Ganoderma polysaccharides[23-25]. GK:葡萄糖激酶;GPI:6-磷酸葡萄糖异构酶;PGM:磷酸葡萄糖变位酶;PMI:甘露糖-6-磷酸异构酶;RHS:鼠李糖合成酶系;RFFH:dTDP-葡萄糖合成酶;UGDH:尿苷二磷酸葡萄糖-6-脱氢酶;UGP:UDP-葡萄糖焦磷酸化酶;UGD:尿苷二磷酸葡萄糖醛酸脱羧酶;PMM:磷酸甘露糖变位酶;GALE:UDP-葡萄糖4-差向异构酶;GFS:GDP-L-岩藻糖合成酶;GMP:GDP-甘露糖焦磷酸化酶;GMDH:GDP-甘露糖4, 6-脱水酶 GK: Glucokinase; GPI: Glucose-6-phosphate isomerase; PGM: Phosphoglucomutase; PMI: Mannose-6-phosphate isomerase; RHS: Rhamnose synthases; RFFH: dTDP-glucose synthase; UGDH: UDP-glucose-6-dehydrogenase; UGP: UDP-glucose pyrophosphorylase; UGD: UDP-glucuronate decarboxylase; PMM: Phosphomannomutase; GALE: UDP-glucose 4-epimerase; GFS: GDP-L-fucose synthase; GMP: GDP-mannose pyrophosphorylase; GMDH: GDP-mannose 4, 6-dehydratase. |
|
|
Ma等[23]首次构建了灵芝菌株CGMCC5.26的基因组规模代谢模型(genome-scale metabolic model,GSMM),完整地阐明了胞外多糖的前体核苷酸糖生物合成途径,并提出了影响胞外多糖高产的8个关键酶基因,分别为葡萄糖磷酸变位酶(PGM)基因、UDP-葡萄糖焦磷酸化酶(UGP)基因、GDP-岩藻糖合成酶(GDP-L-fucose synthase,TSTA3)基因、GDP-甘露糖4, 6-水解酶(GDP-mannose 4, 6-dehydratase,GMDS)基因、UDP-阿拉伯糖4-差向异构酶(UDP-glucuronate decarboxylase,UXE)基因、dTDP-4-脱氢-6-脱氧-D-葡萄糖3, 5-异构体酶(dTDP-L-rhamnose biosynthesis,RFBC)基因、dTDP-葡萄糖4, 6-水解酶(dTDP-L-rhamnose biosynthesis,TGDS)基因和dTDP-葡萄糖合成酶(dTDP-glucose synthase,RFFH)。Tang等[33]发现在一定的培养条件下,灵芝中PGM的活性和胞外多糖的产量具有一定的相关性。Xu等[32]研究了磷酸葡萄糖变位酶(PGM)基因过表达对胞内多糖含量、胞外多糖产量和多糖生物合成相关酶葡萄糖磷酸变位酶(PGM)、UDP-葡萄糖焦磷酸化酶(UGP)和β-1, 3-葡聚糖合成酶(GLS)的基因转录水平的影响,结果表明,高表达PGM基因的灵芝的最大胞内多糖(intracellular polysaccharides,IPS)含量和胞外多糖(extracellular polysaccharides,EPS)产量分别为23.67 mg/100 mg和1.76 g/L,分别比野生型菌株提高40.5%和44.3%;PGM、UGP和GLS的转录水平分别上调了4.77、1.51和1.53倍,这表明多糖生物合成的增加可能是这些基因的高表达所致。Ji等[34]研究了尿苷二磷酸葡萄糖焦磷酸化酶(UGP)基因过表达对胞内多糖含量、胞外多糖产量及磷酸葡萄糖变位酶(PGM)、UDP-葡萄糖焦磷酸化酶(UGP)和α-1, 3-葡聚糖合成酶(GLS)这3个参与多糖生物合成的基因转录水平的影响,发现高表达UGP基因的灵芝最大胞内多糖(IPS)含量和胞外多糖(EPS)产量分别为24.32 mg/100 mg和1.66 g/L,分别比野生型菌株提高了42%和36%;在工程菌株中,PGM、UGP和GLS的转录水平分别上调了1.6、2.6和2.4倍,这表明UGP基因的过表达会导致UDP-葡萄糖的积累增加,可能会刺激参与多糖生物合成的GLS基因的转录。赵丽娜等[35]采用过表达GDP-D-甘露糖焦磷酸化酶(GMP)基因来提高灵芝多糖产量,结果表明,与野生菌株相比,GMP菌株的胞外多糖(EPS)产量及胞内多糖(IPS)含量最高达到了0.991 g/L和21.59 mg/100 mg,比野生菌株分别提高了21.1%和19.5%;GMP基因的过表达也提高了甘露糖磷酸变位酶1 (phosphomannomutase 1,Pmm1)和甘露糖磷酸变位酶2 (phosphomannomutase 2,Pmm2)基因的表达,与野生菌株相比,GMP菌株中Pmm1以及Pmm2基因的表达水平分别上调了2.87倍和2.55倍。Wang等[36]在灵芝中克隆了血红素加氧酶基因(heme oxygenase1,HMX1),并获得了转化子HMX1i1和HMX1i2,与对照组相比,含有HMX1i1和HMX1i2的灵芝胞外多糖含量分别提高了59%和51%,胞内多糖含量分别提高了24%和22%。Li等[37]利用透明颤菌血红蛋白(Vitreoscilla hemoglobin,VHb)基因的表达来提高灵芝多糖产量,发现含有VHb基因的灵芝最大胞内多糖含量和胞外多糖产量分别为26.4 g/100 mg和0.83 g/L,比野生型菌株提高30.5%和88.2%;此外,在含有透明颤菌血红蛋白基因的灵芝中,PGM、UGP和GLS的转录水平分别上调了1.51、1.55和3.83倍。张奇茹等[38]通过添加不同浓度的代谢中间产物葡萄糖-1-磷酸,研究其对灵芝胞外多糖产量、单糖组成、合成途径相关酶的影响,结果表明葡萄糖-1-磷酸的添加对灵芝多糖产量和多糖的单糖组成并无明显影响,但会抑制磷酸葡萄糖变位酶(PGM)、磷酸葡萄糖异构酶(PGI)的酶活;此外,发现发酵过程中半乳糖和甘露糖比例的变化受PGM和PGI、PMI的影响。陈云等[39]探究了葡萄糖基转移酶基因GL24971的过表达对灵芝多糖的影响,发现转化菌株T1与T2的单位菌体胞外多糖最大产量为0.07 mg/mg和0.06 mg/mg,与对照组相比分别提高了132.4%和70.0%,而胞内多糖产量均为0.03 mg/mg,分别降低了10.7%和37.4%,生物量降低了37.5%,为4.16 mg/mL;此外,几丁质和β-1, 3-葡聚糖含量降低,单糖组成中半乳糖的比例减少、甘露糖的比例增加,但仍然以葡萄糖为主。多糖代谢途径中不同酶基因过表达对液态发酵灵芝胞内外多糖的影响如表 1所示。
| 菌株名称 Strain name |
发酵规模 Fermentation scale |
研究内容 Research content |
生物量 Biomass (g/L) |
胞内多糖含量及提高率 Intracellular polysaccharide content and increase rate |
胞外多糖得率及提高率 Yield and increase rate of extracellular polysaccharide |
参考文献 References |
| G. lucidum CGMCC 5.616 | 250 mL flask | Overexpression of α-PGM gene | 8.31 | 23.67 mg/100 mg, increase 40.5% | 1.76 g/L, increase 44.3% |
[32] |
| G. lucidum CGMCC 5.616 | 250 mL flask | Overexpression of UGP gene | 8.08 | 24.32 mg/100 mg, increase 42.0% |
1.66 g/L, increase 36.0% |
[34] |
| G. lucidum CGMCC 5.616 | − | Overexpression of GMP gene | 5.71 | 21.59 mg/100 mg, increase 19.5% |
0.991 g/L, increase 21.1% |
[35] |
| G. lucidum ACCC 5.616 | 250 mL flask | Heterologous expression of VHb gene | 8.13 | 26.40 mg/100 mg, increase 30.5% |
0.83 g/L, increase 88.2% |
[37] |
| G. lucidum 5.26 | − | Overexpression of GL24971 gene | 4.16 | 0.03 mg/100 mg, decrease 10.7% |
0.07 mg/100 mg, increase 132.4% |
[39] |
| 注:α-PGM:α-葡糖磷酸变位酶;UGP:同源尿苷二磷酸葡萄糖焦磷酸化酶;GMP:GDP-D-甘露糖焦磷酸化酶;VHb:透明颤菌血红蛋白;GL:Ganoderma lucidum;−:文献未报道 Note: α-PGM: α-phosphoglucomutase; UGP: Homologous uridine diphosphate glucose pyrophosphorylase; GMP: GDP-D-mannose pyrophosphorylase; VHb: Vitreoscilla hemoglobin; GL: Ganoderma lucidum; −: References unreported. |
||||||
灵芝液态发酵的培养基成分主要包括碳源、氮源、无机盐、生长因子等。培养基的组成不仅影响灵芝液态发酵菌丝体形态及胞内外多糖的产量,而且可以改变胞内外多糖单糖组成、糖链结构和分子量分布,进而影响其生物活性。此外,培养基的优化是灵芝液态发酵和规模化生产的第一步,菌株的特异性使得菌株对碳源、氮源、无机盐的利用及代谢物的积累产生不同的结果,不同培养基组分对灵芝液态发酵胞内外多糖产量及多糖分子量的影响如表 2所示。
| 菌株名称 Strain name |
发酵规模 Fermentation scale |
研究内容 Research content |
生物量 Biomass (g/L) |
胞内多糖含量/得率 Intracellular polysaccharide content/yield |
胞外多糖含量/产量 Exopolysaccharide content/yield |
多糖分子量 Polysaccharide molecular weight |
参考文献 References |
| G. lucidum G0119 | 250 mL摇瓶 250 mL flask 5 L发酵罐 5 L fermenter 50 L发酵罐 50 L fermenter |
Glucose 40 g/L, yeast powder 12 g/L, KH2PO4 3 g/L, initial pH 5.5, inoculum size 10 mL/100 mL | − 25.31 25.20 |
1.98 g/L 2.59 g/L 2.65 g/L |
IPS: 4.695×106 Da EPS: 5.554×104 Da |
[40] | |
| G. cantharelloideum HPMCC 2951901-2# | 5 L发酵罐 5 L fermenter |
Glucose 30 g/L, tryptone 3 g/L, 26 ℃, pH 5.0 | − | − | 1.60 g/L | EPS1: 627.72 kDa EPS2: 74.56 kDa |
[41] |
| G. lucidum CCRC36041 | 250 mL摇瓶 250 mL flask |
Glucose 40 g/L, corn steep powder 5 g/L | 5.58 | − | 1.39 g/L | EPS: 71−209 kDa | [42] |
| G. lucidum | 摇瓶 flask |
Fe2+ 0.2% | 7.299 | − | 0.72 g/L | − | [43] |
| G. lucidum GL-01 | 500 mL摇瓶 500 mL flask |
硬脂酸 Stearic acid 亚油酸 Linoleic acid |
0.99 1.06 |
− |
1.06 g/L 0.77 g/L |
[44] | |
| G. lucidum CCRC36123 | 500 mL摇瓶 500 mL flask |
玉米油 Corn oil |
12.90 | 1.038 g/L | [45] | ||
| G. lucidum WZ06 | 500 mL摇瓶 500 mL flask |
薏苡仁油 Coix lacryma-jobi oil |
10.71 | 0.38 g/L | 0.33 g/L | [46] | |
| G. lucidum CGMCC5.26 | 500 mL摇瓶 500 mL flask |
L-苯丙氨酸 L-phenylalanine |
− | − | 0.91 g/L | [47] | |
| G. resinaceum YW01 | 250 mL摇瓶 250 mL flask |
Tween-80 | 7.24 | − | 1.33 g/L | EPS: 4.61×105 Da | [48] |
| G. lucidum | − | 木素木霉激发子 Trichoderma viride elictor 顶头孢激发子 Acremonium strictum elictor |
7.53 − |
− 0.370 g/L |
− − |
[49] | |
| G. lucidum Gl-01 | 500 mL摇瓶 500 mL flask |
稀土元素镨 Rare earth elements Pr3+ |
− | 772.56 mg/L | 452.76 mg/L | [50] | |
| G. lucidum | 500 mL摇瓶 500 mL flask |
药用昆虫蜣螂 Medicinal insect Catharsius molossus |
− | 2.41 g/L | 608.90 mg/L | [51] | |
| G. lucidum 5.534 | 1 000 L摇瓶 1 000 mL flask |
甘草 Licorice |
18.84 | 3.61 g/L | 6.21 g/L | [52] | |
| 注:−:文献未报道 Note: −: References unreported. |
|||||||
碳源是构成细胞物质的基本元素,为微生物提供合成产物的碳架及生长发育所需的基本能量。碳源有单糖、双糖和多糖,例如葡萄糖、蔗糖、麦芽糖、乳糖、玉米粉、淀粉等。有研究表明,在灵芝多糖合成方面,碳源中葡萄糖、可溶性淀粉更为适合。Zapata等[53]分别以大麦粉、燕麦粉、小麦粉、玉米粉、大米粉、小米粉及以上等量混合粉为碳源,研究了非常规碳源对菌丝体生物量以及多糖含量的影响,发现最佳碳源为大麦粉且浓度为50 g/L、pH值为6.0时,生物量最高为(23.49±0.37) g/L;pH值为5.5时,胞内多糖含量最高为(2.72±0.11) g/L,胞外多糖含量最高为(2.22±0.06) g/L。Feng等[40]探究了不同碳源对胞内多糖产量的影响,发现影响胞内多糖产量的碳源由高到低依次为葡萄糖、蔗糖、羧甲基纤维素和淀粉;当葡萄糖浓度为20 g/L时,胞内多糖得率最高为1.12 g/L。Long等[41]对比了7种碳源对胞外多糖产量的影响,发现以葡萄糖为碳源时,生物量和EPS产量最高分别为6.53 g/L和1.90 g/L。张冬雪等[54]发现当碳源为可溶性淀粉时,β‐葡聚糖得率、多糖得率及生物量明显高于其他碳源。Peng等[55]研究了混合碳源对灵芝胞外多糖的单糖组成及相关酶磷酸葡萄糖异构酶(PGI)、磷酸葡萄糖变位酶(PGM)、磷酸甘露糖异构酶(PMI)和GDP-D-Man焦磷酸化酶(GMP)活性的影响,发现EPS单糖的结合摩尔百分比与碳源中相同,均大于80%;PGI、PGM、PMI、GMP的活性受混合碳源中单糖比例及摩尔百分比的影响;此外,观察到不同碳源条件下关键合成酶活性和编码基因表达水平的差异,提示灵芝多糖的单糖组成可能控制在翻译水平。李洁等[56]研究了混合碳源对灵芝发酵及抗肿瘤活性的影响,发现不同比例的单糖碳源对灵芝多糖产量和生物量影响较小,碳源比例对灵芝多糖活性影响较大;葡萄糖和半乳糖以质量浓度比1:1作碳源时抑制率可达到50%−60%,葡萄糖、半乳糖和甘露糖质量浓度比1:1:1作碳源时抑制率达到80%−85%;初始培养基中半乳糖和甘露糖所占比例大有利于灵芝多糖抗肿瘤活性的发挥。
3.2 氮源氮源是微生物发酵中核酸、蛋白质、酶等生物合成的主要原料,对微生物的生长发育起着重要作用。氮源有无机氮源和有机氮源,例如硫酸铵、氯化铵、蛋白胨、酵母粉、豆饼粉等。有研究表明,在灵芝多糖合成方面,有机氮源中豆粕粉、酵母粉、蛋白胨更适合。钱磊等[57]探讨了8种氮源对灵芝胞外多糖产量的影响,发现有机氮源和无机氮源均可被菌丝体利用;当有机氮源为豆饼粉时胞外多糖产量最高,为129.1 mg/100 mL。Long等[41]对比了6种氮源对胞外多糖产量的影响,发现当氮源为蛋白胨时,EPS产量最高,为1.87 g/L,但生物量较低;而以酵母粉为氮源时,生物量最高,为5.93 g/L。这表明,合成EPS营养的需求可能与菌丝体生物量不一致。Feng等[40]发现当以酵母粉和蛋白胨为氮源时胞内多糖产量较高,二者无显著性差异;当酵母粉浓度为12 g/L时,胞内多糖得率最高为1.30 g/L。张冬雪等[54]发现有机氮源作为氮源时的β-葡聚糖得率优于无机氮源,而且复合氮源并非最利于多糖的合成,当蛋白胨作为单一氮源时β-葡聚糖的得率最高。Hsieh等[42]研究了限制氮源情况下对胞外多糖产量及多糖分子量的影响,结果表明氮源限制条件下多糖产量较高,但氮源限制条件下多糖相对分子质量较低,玉米浆干粉(corn steep powder,CSP)为1、2和5 g/L时多糖分子量分布分别为61−90、67−113和71−209 kDa。Fraga等[58]发现培养基中较低的蛋白胨浓度(1.65 g/L)有利于提高菌丝生物量和胞外多糖的纯度,而较高的蛋白胨水平(4.80 g/L)能最大限度地提高胞外多糖产量;此外,高浓度的葡萄糖及pH为4.0、6.0条件下有利于形成(1→3)/(1→4)-Glcp比值较高的胞外多糖,较低的蛋白胨浓度及pH为5.0条件下有利形成分支度较高的胞外多糖。
3.3 无机盐及生长因子无机盐及生长因子在微生物细胞的生理和代谢过程中起着重要的作用。例如:作为酶的激活剂参与糖的代谢过程(K+、Mg2+);维持细胞内的酸碱平衡(Ca2+);促进菌体生长及产物的合成等(B族维生素)。Feng等[40]对比了KH2PO4、K2HPO4、Na2HPO4、NaH2PO4对灵芝胞内多糖得率的影响,发现KH2PO4最适合菌丝体合成胞内多糖,当KH2PO4浓度为2.5 g/L时胞内多糖得率最高为1.19 g/L,而Na2HPO4、NaH2PO4培养条件下菌丝体生长缓慢,多糖产生较少。张冬雪等[54]发现当培养基中无机盐为K2HPO4时,β-葡聚糖得率最高,而且多糖得率明显高于MgSO4、CaCl2、K2HPO4等无机盐;当K2HPO4浓度为1.5 g/L时,β-葡聚糖得率及多糖含量均达最大。叶盛权等[43]对比了含有K+、Mg2+、Zn2+、Fe2+、Ca2+的培养基对灵芝液态发酵多糖含量的影响,发现含Fe2+发酵培养基的生物量和胞外粗多糖的量最高,分别为7.299 g/L和0.720 g/L,在同等条件下进行发酵罐扩大培养,所得到的胞外粗多糖含量比摇瓶发酵培养所得略高。姚强等[50]研究了稀土元素(镧(La)、铈(Ce)、钕(Nd)、镨(Pr)、铒(Er))对灵芝液体发酵的影响,发现不同的稀土元素在适宜的浓度下均有提高灵芝多糖产量的作用;其中镨元素对灵芝胞内多糖和胞外多糖生产促进作用最明显,当浓度为0.01、0.001 mmol/L时二者产量分别达到最高,是对照组的4.67、3.05倍。曾晓希等[59]研究了维生素B1、B2和B6作为生长因子对灵芝多糖液态发酵的影响,结果表明,3种维生素B均能促进灵芝的生长和胞外多糖的积累;其中维生素B1效果最好,生物量和胞外多糖分别达到7.604 g/L和0.863 g/L。
3.4 外源添加物近年来,在优化培养基的基础之上,许多学者采用添加外源物对发酵过程进行诱导、干预和调控进一步来提高胞内外多糖的产量。外源添加物主要包括中药、油脂类物质、信号分子等。灵芝可依靠自身的酶系对中药中的营养物质进行转化和利用,促进自身的生长及代谢产物的合成。油脂类物质不仅可以作为消泡剂来影响液态体积中氧的传递,还可以起到保护细胞、改变细胞膜的通透性及影响代谢中相关酶的活性等来提高多糖的产量。信号分子则可通过激活特定代谢途径来提高产物的产量。姚强等[44]研究了不同脂肪酸对灵芝液体发酵的影响,结果表明,硬脂酸、亚油酸和棕榈酸都能提高灵芝菌丝体生物量;硬脂酸的添加有利于灵芝胞外多糖的产生,当添加浓度为1.0 g/L时,胞外多糖含量是对照组的3.37倍;亚油酸的添加有利于胞内多糖的合成,当添加浓度为1.5 g/L,胞内多糖含量是对照组的2.56倍。Huang等[45]研究了添加玉米油对灵芝菌丝生长和多糖合成的影响,在培养初期添加2%玉米油,菌体生物量和多糖产量在培养13 d时分别达到最大值12.9 g/L和1.038 g/L,与对照组相比,菌体生物量提高了2.35倍;胞外多糖产量提高了1.78倍;对菌体形态分析发现,油脂可以间接保护细胞在低pH条件下缓慢裂解。此外,玉米油可通过提高葡萄糖转化效率来促进生物量和胞外多糖的产生。Yang等[46]在培养开始时添加2%的薏苡仁油时,生物量、胞外多糖和胞内多糖的产量分别达到最大值,分别是对照组的3.34、2.76、2.20和2.23倍;酶活性分析表明,油脂或其脂肪酸通过直接影响不同时期磷酸葡萄糖异构酶和β-磷酸葡萄糖变位酶的合成水平来发挥作用。Yang等[48]研究了Tween-80对灵芝深层发酵生产灵芝胞外多糖(EPS)的促进作用,结果表明,添加0.25% Tween-80的菌丝生物量和胞外多糖产量在第3天最高,分别提高了19.76%和137.50%;发酵动力学分析表明,加入Tween-80后葡萄糖消耗加快,EPS生物合成相关基因的表达和ATP生成显著提高;此外,Tween-80还显著增加了细胞内活性氧的积累,增加了细胞膜和细胞壁的通透性;与不含Tween-80的EPS相比,含Tween-80的EPS具有更高糖醛酸含量、更低的分子量,以及更高的抗氧化活性,对2, 2-二苯基-1-苦基肼(DPPH)自由基的抗氧化能力更强。Ma等[47]研究了L-苯丙氨酸对灵芝胞外多糖(EPS)的影响。当L-苯丙氨酸浓度为0.4 g/L时,培养24 h后EPS产量达到0.91 g/L,比对照(0.56 g/L)提高62.5%;转录组和基因组分析表明,与L-苯丙氨酸代谢相关的L-苯丙氨酸脱氨酶和苯甲酸4-羟化酶显著上调,而细胞壁甘露糖蛋白基因下调;透射电子显微镜(TEM)和原子力显微镜结果表明,L-苯丙氨酸处理的细胞壁厚度减少了58.58%,细胞壁孔隙率增加,这可能是提高EPS产量的原因之一;L-苯丙氨酸及其代谢中间体能促进IPS的产生,对灵芝细胞壁的合成有明显的抑制作用。高兴喜[49]等以3种食用菌病原真菌(木素木霉、蘑菇轮枝孢和顶头孢)为激发子菌株分别制备相应激发子,以不同浓度和不同时间添加到灵芝发酵培养基中,发现顶头孢激发子对灵芝多糖的诱导作用最好,添加时间为灵芝培养的初期(0 d)和中期(3 d)、浓度为120 μg/mL时诱导效果最佳,多糖产量达到357.28 mg/L,是对照组的5.8倍。刘高强等[51]研究了几种药用昆虫对灵芝多糖生物合成的影响,发现昆虫蜣螂添加浓度为5 g/L时能显著促进灵芝IPS和EPS的形成,其产量分别由对照组的(1.93±0.09) g/L和(520.3±20.2) mg/L提高到(2.41±0.12) g/L和(608.9±20.2) mg/L。朱强等[52]在液体培养基中分别加入甘草、山茱萸、黄芪、黄岑4种中药,发现甘草和山茱萸对灵芝多糖的产生有明显的促进作用;胞内多糖和胞外多糖均在甘草添加量为6 g/L时达到最大值为3.613 g/L和6.217 g/L,分别比空白组提高了70.42%和61.44%。薛菲菲等[60]探究了14种外源物质对灵芝发酵合成β-葡聚糖的影响,结果表明,薏苡仁酯可定向诱导灵芝β-葡聚糖的合成,在最优条件下,β-葡聚糖的产量可达(40.67±8.43) mg/L。
4 发酵工艺条件对灵芝液态发酵胞内外多糖的影响灵芝液态发酵工艺条件包括pH、温度、光照、通气量、发酵方式及其他处理等。不同的发酵条件会改变菌株在代谢过程中发酵液的流变特性、营养物质的传递吸收、菌丝体形态、细胞的状态、酶的活性以及生长环境等。此外,发酵条件不只对代谢产物的合成和富集产生影响,还使得代谢产物多糖的分子量、化学组成、分支方式及构象发生变化,从而表现出不同的生物活性。发酵工艺条件对灵芝液体发酵胞内外多糖产量的影响如表 3所示。
| 菌株名称 Strain |
发酵规模 Fermentation scale |
研究内容 Research content |
生物量 Biomass (g/L) |
胞内多糖含量/得率 Intracellular polysaccharide content/yield (g/L) |
胞外多糖含量/产量 Exopolysaccharide content/yield (g/L) |
参考文献References |
| G. lucidum DG-6566 | 5 L发酵罐 5 L fermenter |
多阶段控制pH Multi-stage pH control |
12.50 | − | 4.70 | [61] |
| G. lucidum | 500 mL摇瓶 500 mL flask |
温度30 ℃ Temperature 30 ℃ |
4.18 | 0.48 | [62] | |
| G. lucidum CCGMC5.616 | 3.5 L发酵罐 3.5 L fermenter |
初始体积氧传递系数(KLa) 为78.2 h−1 An initial KLa of 78.2 h−1 |
15.62 | 2.19 | − | [63] |
| G. resinaceum DG-6566 | 5 L发酵罐 5 L fermenter |
补料分批发酵,初始葡萄糖浓度10 g/L,第6天补料葡萄糖浓度50 g/L Fed-batch fermentation, initial glucose concentration 10 g/L, fed glucose concentration 50 g/L on the sixth day |
42.20 | − | 4.60 | [64] |
| G. lucidum CCRC36123 | 15 L发酵罐 15 L fermenter |
补料分批发酵中控制溶氧(DO) 20%以上 Control of dissolved oxygen (DO) over 20% in fed-batch fermentation |
26.60 | − | 4.55 | [65] |
| G. lucidum CGMCC5.616 | 200 L发酵罐 200 L fermenter |
转速、pH、溶氧和补料分批发酵相结合 Combined regulation of rotational speed, pH, dissolved oxygen and fed-batch fermentation |
22.62 | 4.74 | − | [66] |
| G. lucidum xy05 | 10 L发酵罐 10 L fermenter |
DO 0−56 h为30%;56−112 h为20%;112−168 h为10% DO was 30% in 0−56 h; 20% for 56−112 h; 10% for 112−168 h |
10.88 | 2.88 | [67] | |
| G. lucidum G0119 | 5 L发酵罐 5 L fermenter |
0−31.2 h通气量为6 L/min;31.2−43 h通气量为9 L/min;43−55 h通气量为6 L/min;55−96 h (发酵结束)通气量为5 L/min | 17.35 | 2.83 | − | [68] |
| 0−31.2 h ventilation rate was 6 L/min; 31.2−43 h ventilation was 9 L/min; the ventilation rate was 6 L/min in 43−55 h. Ventilation at 55−96 h (end of fermentation) 5 L/min | ||||||
| G. lucidum G9 | 500 mL摇瓶 500 mL flask |
100 μE蓝光LED光照10 h 100 μE Blue LED for 10 h |
15.16 | − | 3.10 | [69] |
| G. lucidum G10016 | 250 mL摇瓶 250 mL flask |
超声波均质菌丝体作为接种物 Ultrasonic homogeneous mycelium as inoculum |
15.75 | − | 0.54 | [70] |
| G. lucidum CGMCC 5.0026 | 250 mL摇瓶 250 mL flask |
超声处理时间15 min,功率15 W/L,处理次数2次 The ultrasonic treatment time was 15 min, the power was 15 W/L, and the treatment times were 2 times |
15.54 | 0.18 | 0.92 | [71] |
| G. lucidum CCGMC 5.616 | 250 mL摇瓶 250 mL flask |
三阶段光照射、多次铜离子及补料分批发酵相结合 Multi-fed batch culture integrated with three-stage light irradiation and multiple additions of copper ions |
23.41 | 3.35 | 2.08 | [72] |
| 注:DO:Dissolved oxygen;−:文献未报道 Note: DO: Dissolved oxygen; −: References unreported. |
||||||
液态发酵过程中,培养温度可通过影响灵芝生长及多糖合成过程中相关酶的活性,进而影响细胞对营养物质的吸收利用和产物的合成。Balamurugan等[73]考虑到不同气候地域条件下的灵芝生长的最佳温度不一样,经实验发现低温(20 ℃)条件下,灵芝遵循传统真菌的生长曲线模式;温度为25 ℃和30 ℃条件下会产生不规则的生长曲线模式。Lee等[74]发现温度为25 ℃时,灵芝胞外多糖的产量最高;温度为10 ℃时,胞内多糖和生物量含量最高。刘月芹等[75]研究了提高培养温度对灵芝多糖含量的影响,发现灵芝多糖的含量随着温度的升高呈现先升高后降低的趋势,当发酵温度为34 ℃时,多糖含量最高值为2.66 g/L。刘月芹等[76]发现较高的温度虽然不利于菌丝体生物量的积累,但可提高淀粉酶﹑漆酶﹑纤维素酶等胞外酶的活性;相比30 ℃培养条件下,34 ℃条件下发酵10 d,漆酶活性、纤维素酶活性、可溶性糖含量分别提高了51.12%、30.41%、13.21%。乔双逵等[62]发现液态发酵过程中不同培养温度条件下获得的胞外多糖会影响其对小鼠肝癌细胞Hepa1-6和人乳腺癌细胞MDA-MB-231具有抑制作用;当培养温度为30 ℃时,所得胞外多糖产量和菌体生物量均为最大值,分别为0.487 g/L和4.18 g/L,对肿瘤细胞的抑制率最大。Peng等[77]发现灵芝胞外多糖中单糖的摩尔百分含量受培养温度的影响,其中葡萄糖的摩尔百分含量大于85%并随培养温度的上升呈下降趋势,而甘露糖和半乳糖的摩尔百分含量增加;此外,在不同培养温度条件下,参与胞外多糖合成的9种酶中PGM、UGPase、PGI和PMI四种酶的活性都保持了高于其他酶活的水平。PGM、PGI、GMP三种酶的最适培养温度为℃,PMI为25 ℃,其他酶的最适温度均为30 ℃。
4.2 光照光照对细胞生长和代谢产物的生物合成可能因光质不同而不同。Zapata等[78]探究了4种不同光照及黑暗处理对菌丝体生物量的影响,结果表明,在蓝光处理条件下,灵芝菌丝体生物量最高为(18.66±0.812) g/L。Zhang等[79]对比了3种光源及黑暗处理对灵芝生长及多糖合成的影响,发现在白光照射下,菌丝体生物量、IPS含量和产量最高,分别为(15.36±0.58) g/L、(17.60±0.30) mg/100 mg和(2.70±0.18) g/L。张业尼等[69]研究单色LED光照处理对灵芝液态发酵合成胞外多糖的影响,发现白光LED最有利于细胞生长;蓝光LED最有利胞外多糖的合成,以100 μE蓝光LED光照10 h,生物量和胞外多糖产量分别为15.16 g/L和3.102 g/L,比黑暗处理分别提高了45.91%和109.74%。Zhu等[72]采用三阶段光照射、多次铜离子及补料分批发酵相结合的方式提高IPS含量,与多次添加铜离子、三阶段光照射和多次补料分批发酵相比,IPS含量分别提高了2.28、1.08和1.14倍。余吴梦晓等[80]发现采用蓝光培养灵芝8 d,灵芝多糖含量和己糖激酶(hexokinase,HK)活性相比黑暗对照组分别高了13%和40%,而葡萄糖-6-磷酸脱氢酶(G-6-PDH)活性、磷酸葡萄糖异构酶(PGI)活性和α-磷酸葡萄糖变位酶(α-PGM)活性分别是黑暗对照组的1.75、1.58和1.4倍。
4.3 pHpH在液态发酵过程中的影响是多方面的,例如影响细胞膜的功能、细胞的形态、基质的离子状态、酶的活性、营养物质的吸收和产物的生成等。Fang等[81]发现在液态发酵过程中,较高的初始pH 6.5条件下,细胞呼吸活性较高,细胞生长较好,而且葡萄糖的消耗速率随培养基初始pH增加而增加,初始pH值为3.5、4.5和6.5时葡萄糖每日消耗速率分别为2.34、3.14和3.51 g/L;在低初始pH条件下,残留葡萄糖水平较高时,EPS的产生随发酵时间延长而增加;而较高的初始pH值(5.5−7.0)有利于IPS的生产和产量。Kim等[61]采用两阶段控制pH策略来最大化菌丝体生物量和胞外多糖的产量,并分析了pH对胞外多糖的影响;结果表明两阶段pH控制条件下菌丝体生物量和胞外多糖产量最高值分别为12.5 g/L和4.7 g/L,比不控制pH条件下提高了23%和160%;发现不同pH控制条件下,菌丝体的形状不一,菌球分散程度越高,越有利于胞外多糖的产生,所得到的胞外多糖的分子量、单糖比例和化学组成均有显著性差异。乔双逵等[62]研究了培养基初始pH值对灵芝菌丝体形态及胞外多糖活性的影响,发现较高的初始pH值(5.0−7.0)有利于胞外多糖的合成,较低pH值(3.0−4.0)有利于菌体的生长,中小型菌球(直径D < 2.5 mm)有利于胞外多糖的生成,而大型菌球不利于胞外多糖的生成;培养基初始pH值为4.0条件下获得的胞外多糖对小鼠肝癌细胞Hepa1-6和人乳腺癌细胞MDA-MB-231具有更高的抑制作用。Peng等[77]发现灵芝胞外多糖中葡萄糖的摩尔百分含量随初始pH值的升高呈上升趋势,半乳糖的摩尔百分含量呈下降趋势;而与胞外多糖相关合成酶则表现出一致性,初始pH值越高其酶的活性越低。
4.4 溶氧灵芝属于好氧真菌,液态发酵过程中溶氧会对灵芝菌丝体的生长和活性物质合成产生较大的影响,灵芝生长的不同阶段对氧的需求不同,而溶氧通常与通气量和转速有着密不可分的联系。Tang等[63]研究了初始体积氧传递系数(KLa)和溶解氧张力(DOT)对灵芝发酵的影响,发现在初始KLa为78.2 h−1的条件下,最大生物量为15.62 g/L,最大胞内多糖产量为2.19 g/L;10% DOT条件下胞内外多糖含量高于25% DOT控制条件下,但总产量要低于25% DOT控制条件下。周小苹等[67]研究了摇瓶转速和装液量与氧容积传递系数的关系,进一步在发酵罐上比较了恒定发酵罐搅拌转速、恒定发酵液DO值和分段控制发酵液DO值3种不同控氧方式下对菌丝生长和产胞外多糖的影响,结果表明氧容积传递系数控制在38−68 h−1时,发酵效果最佳;3种不同控氧方式中,分段控制发酵液DO值效果最佳,最优条件下菌丝干重达10.88 g/L,胞外多糖达2.88 g/L。吴迪等[68]在5 L发酵罐中探究了4−10 L/min不同通气量对灵芝菌丝体生长和胞内多糖合成的影响,发现通气量9 L/min时菌丝体干重最高为15.42 g/L;通气量6 L/min时胞内多糖得率最高为2.10 g/L,并依据菌丝生长速率及胞内多糖合成速率,提出了四阶段通气量调控策略,不仅使得生物量和胞内多糖得率分别提高了12.59%和35.75%,而且有着重要的工业应用价值和潜力。
4.5 发酵方式发酵方式主要有连续发酵、分批发酵、补料分批发酵和重复分批发酵等。张雪洪等[82]采用膜生物反应器系统进行灵芝多糖的连续发酵,发现菌丝体总生物量、多糖总产量要比间歇发酵高。Kim等[64]采用补料分批发酵培养来提高菌丝体生物量和胞外多糖的产量,第6天将浓度为50 g/L葡萄糖补料到初始10 g/L葡萄糖培养基中,发酵第14天时菌丝体生物量最大值为42.2 g/L、胞外多糖产量最大值为4.6 g/L。Fazenda等[65]研究了相对溶氧(dissolved oxygen, DO)对灵芝补料分批发酵过程中菌丝体形态和发酵液流变特性的影响,结果表明,当控制补料分批发酵中氧饱和度超过20%可使得生物量提高130%,而不控制DO情况下,胞外多糖产量是控制DO条件下的2倍。Tang等[66]在低转速下控制pH、溶氧并结合补料发酵,成功将发酵规模逐步从7.5 L扩大到20 L、200L发酵罐,并维持产物趋势的稳定性,在最优条件下,最大生物量为22.62 g/L、胞内多糖含量为4.74 g/L。Wan Abd等[83]采用重复分批发酵的方式,在缩短了发酵周期的同时并提高了胞外多糖的产率。
4.6 其他处理Liu等[70]采用超声波来均质菌丝体作为接种物进行深层培养,当接种量为4.76% (体积分数)时,生物量和EPS产量分别达到15.75 g/L和0.54 g/L,分别比对照组提高129.26%和45.95%;此外,超声波处理会导致某些蛋白质等物质外泄以及酶活和代谢活性的降低,但也显著降低了接种量。Sun等[71]研究了低强度超声波对灵芝液态发酵生物量和代谢产物的影响,并对超声条件进行了优化,最佳条件为处理时间15 min,超声功率密度15 W/L,处理次数2次;在最优条件下,与对照组相比,生物量、胞内和胞外多糖含量分别提高了26.99%、18.48%和35.90%。与对照组相比,低强度超声处理改变了灵芝菌丝体结构,提高了细胞内钙离子浓度,提高了细胞膜的通透性,促进了细胞内ROS的积累[71]。Sun等[84]通过转录组和代谢组联合分析了超声波处理对灵芝代谢的影响,结果表明,经超声处理后上调了16个参与多糖合成的基因表达,主要包括木糖还原酶、木糖激酶、UDP-葡萄糖4-差向异构酶糖基转移酶(GT家族)和糖苷水解酶(GH家族)。此外,对初级代谢(氨基酸、糖和脂代谢)、次级代谢(萜类代谢)、细胞膜(转运蛋白和膜组分)和热应激反应产生一定的影响,说明超声处理对灵芝代谢的影响是多方面的[84]。
5 展望灵芝多糖具有广泛的药理活性及悠久的药用历史,在当前绿色、健康、可持续和高质量发展的大环境下,市场对灵芝多糖的需求与日俱增,液态发酵是灵芝多糖来源的重要途径及趋势之一,但仍存在一定局限性。我们认为可以从以下几个方面着手:(1) 灵芝多糖的生物代谢途径中,关于多糖合成前体核苷酸糖单糖的组装及聚合过程尚不清晰,需进一步探究阐明;在液态发酵及调控过程中,需进一步探究发酵条件对灵芝多糖单糖的组成比例、连接方式、糖苷键的支化程度影响的规律。(2) 国内外对液态发酵生产灵芝多糖的规模主要集中在实验室摇瓶阶段,远不能满足市场需求;不同生物反应器中传质、传热及流变学特性之间等差异较大,使得工业规模化大罐发酵与摇瓶发酵产物结果是否具有一致性,有待进一步深入研究。(3) 灵芝共培养是一种诱导和激发灵芝代谢产物、基因表达方式及新化合物产生的途径。有学者通过共培养来提高目标活性多糖的产量;也有学者通过共培养合成一系列木糖苷类化合物;也有学者通过共培养提高某种多糖的药理活性[85-88]。但仍存在一定的不可控性,如所产多糖的原理较难解释和追踪,须进一步深入探究。(4) 灵芝液态发酵过程中合成的多糖种类、结构、分子量分布等受发酵条件影响较大,此外,并不是所有的多糖都具有较好的药理活性,是否可通过发酵工艺调控来达成定向发酵某种生物活性的多糖,简化工业化发酵后期不同多糖分离纯化等过程,增强目标产物多糖的药理活性。(5) 灵芝多糖的分子量大小与其生物活性具有一定的相关性,然而液态发酵工艺研究主要集中在工艺优化来提高生物量、总多糖等方面,未见聚焦在定向生产有针对特定分子量大小的灵芝多糖液态发酵工艺的研究。本研究团队拟针对高分子量灵芝多糖的定向液态发酵展开系统性研究,以期更深一步地探究液态发酵工艺与灵芝多糖分子量之间的关系,为灵芝产业提供定向发酵生产灵芝多糖的技术。
| [1] |
Cui BK, Wu SH. The scientific name of the widely cultivated Ganoderma species[J]. Mycosystema, 2020, 39(1): 7-12. (in Chinese) 崔宝凯, 吴声华. 普遍栽培灵芝种类的拉丁学名[J]. 菌物学报, 2020, 39(1): 7-12. DOI:10.13346/j.mycosystema.190388 |
| [2] |
Lin ZB, Yang BX. Ganoderma and Health[M]. Singapore: Springer Singapore, 2019.
|
| [3] |
Bishop KS, Kao CHJ, Xu YY, Glucina MP, Paterson RRM, Ferguson LR. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals[J]. Phytochemistry, 2015, 114: 56-65. DOI:10.1016/j.phytochem.2015.02.015 |
| [4] |
Hapuarachchi KK, Elkhateeb WA, Karunarathna SC, Cheng CR, Bandara AR, Kakumyan P, Hyde KD, Daba GM, Wen TC. Current status of global Ganoderma cultivation, products, industry and market[J]. Mycosphere, 2018, 9(5): 1025-1052. DOI:10.5943/mycosphere/9/5/6 |
| [5] |
Ahmad MF. Ganoderma lucidum: a rational pharmacological approach to surmount cancer[J]. Journal of Ethnopharmacology, 2020, 260: 113047. DOI:10.1016/j.jep.2020.113047 |
| [6] |
Lu JH, He RJ, Sun PL, Zhang FM, Linhardt RJ, Zhang AQ. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review[J]. International Journal of Biological Macromolecules, 2020, 150: 765-774. DOI:10.1016/j.ijbiomac.2020.02.035 |
| [7] |
Zhang K, Liu YF, Zhao XL, Tang QJ, Dernedde J, Zhang JS, Fan H. Anti-inflammatory properties of GLPss58, a sulfated polysaccharide from Ganoderma lucidum[J]. International Journal of Biological Macromolecules, 2018, 107(Pt A): 486-493. |
| [8] |
Ren L, Zhang J, Zhang TH. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells[J]. Food Chemistry, 2021, 340: 127933. DOI:10.1016/j.foodchem.2020.127933 |
| [9] |
Zhao C, Zhang CC, Xing Z, Ahmad Z, Li JS, Chang MW. Pharmacological effects of natural Ganoderma and its extracts on neurological diseases: a comprehensive review[J]. International Journal of Biological Macromolecules, 2019, 121: 1160-1178. DOI:10.1016/j.ijbiomac.2018.10.076 |
| [10] |
Yu HZ, Liu YF, Zhou S, Yan MQ, Xue LK, Tang QJ, Zhang JS. Comparison of the polysaccharides from fruiting bodies, mycelia and spore powder of Ganoderma lingzhi[J]. Mycosystema, 2016, 35(2): 170-177. (in Chinese) 于华峥, 刘艳芳, 周帅, 颜梦秋, 薛令坤, 唐庆九, 张劲松. 灵芝子实体、菌丝体及孢子粉中多糖成分差异比较研究[J]. 菌物学报, 2016, 35(2): 170-177. |
| [11] |
Li Y, Zhang HN, Tan Y, Liu YF, Feng J, Zhang YY, Tang CH, Zhang JS. Screening of a high polysaccharide content Ganoderma lucidum strain by ARTP[J]. Acta Edulis Fungi, 2021, 28(2): 36-41. (in Chinese) 李塬, 张赫男, 谭贻, 刘艳芳, 冯杰, 张越野, 唐传红, 张劲松. 常压室温等离子体诱变选育高产多糖灵芝新菌株[J]. 食用菌学报, 2021, 28(2): 36-41. DOI:10.16488/j.cnki.1005-9873.2021.02.005 |
| [12] |
Gao K, Feng J, Yan MQ, Tang QJ, Zhang JS, Zhou S, Yang Y, Feng N, Liu YF. Screening of extracellular polysaccharide high-yielding strains of Ganoderma lingzhi in liquid submerged fermentation and polysaccharide characteristic analysis[J]. Mycosystema, 2019, 38(6): 886-894. (in Chinese) 高坤, 冯杰, 颜梦秋, 唐庆九, 张劲松, 周帅, 杨焱, 冯娜, 刘艳芳. 灵芝液态发酵高产胞外多糖菌株筛选及多糖特性分析[J]. 菌物学报, 2019, 38(6): 886-894. |
| [13] |
Feng J, Feng N, Tang QJ, Liu YF, Tang CH, Zhou S, Wang JY, Tan Y, Zhang JS, Lin CC. Development and optimization of the triterpenoid and sterol production process with Lingzhi or reishi medicinal mushroom, Ganoderma lucidum strain G0017 (Agaricomycetes), in liquid submerged fermentation at large scale[J]. International Journal of Medicinal Mushrooms, 2021, 23(3): 43-53. DOI:10.1615/IntJMedMushrooms.2021037830 |
| [14] |
Feng J, Feng N, Zhang JS, Yang Y, Jia W, Lin CC. A new temperature control shifting strategy for enhanced triterpene production by Ganoderma lucidum G0119 based on submerged liquid fermentation[J]. Applied Biochemistry and Biotechnology, 2016, 180(4): 740-752. DOI:10.1007/s12010-016-2129-1 |
| [15] |
Tang CM, Zhang JS, Liu YF, Tang QJ, Feng N, Tang CH, Wang JY, Tan Y, Liu LP, Feng J. Application progress of atmospheric and room temperature plasma mutation breeding and microbial microdroplet culture screening technology[J]. Microbiology China, 2022, 49(3): 1177-1194. (in Chinese) 唐晨旻, 张劲松, 刘艳芳, 唐庆九, 冯娜, 唐传红, 王金艳, 谭贻, 刘利平, 冯杰. 常压室温等离子体诱变育种与微生物液滴培养筛选技术应用进展[J]. 微生物学通报, 2022, 49(3): 1177-1194. |
| [16] |
Wang YT, Liu YF, Yu HZ, Zhou S, Zhang Z, Wu D, Yan MQ, Tang QJ, Zhang JS. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum[J]. Carbohydrate Polymers, 2017, 167: 337-344. DOI:10.1016/j.carbpol.2017.03.016 |
| [17] |
Wang CH, Hsieh SC, Wang HJ, Chen ML, Lin BF, Chiang BH, Lu TJ. Concentration variation and molecular characteristics of soluble (1, 3; 1, 6)-β-D-glucans in submerged cultivation products of Ganoderma lucidum mycelium[J]. Journal of Agricultural and Food Chemistry, 2014, 62(3): 634-641. DOI:10.1021/jf404533b |
| [18] |
Liu YF, Zhang JS, Tang QJ, Yang Y, Guo QB, Wang Q, Wu D, Cui SW. Physicochemical characterization of a high molecular weight bioactive β-D-glucan from the fruiting bodies of Ganoderma lucidum[J]. Carbohydrate Polymers, 2014, 101: 968-974. DOI:10.1016/j.carbpol.2013.10.024 |
| [19] |
Xu Y, Zhang X, Yan XH, Zhang JL, Wang LY, Xue H, Jiang GC, Ma XT, Liu XJ. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum[J]. International Journal of Biological Macromolecules, 2019, 135: 706-716. DOI:10.1016/j.ijbiomac.2019.05.166 |
| [20] |
Sui XC, Guo QB, Xia YM, Cui SW, Shen J, Zhang J, Ding ZY. Structure features of the intracellular polysaccharide from Ganoderma lucidum and the irrelative immune-anticancer activities of GLPs[J]. Bioactive Carbohydrates and Dietary Fibre, 2016, 8(2): 43-50. DOI:10.1016/j.bcdf.2016.11.001 |
| [21] |
Cai M, Xing HY, Tian BM, Xu J, Li ZH, Zhu H, Yang K, Sun PL. Characteristics and antifatigue activity of graded polysaccharides from Ganoderma lucidum separated by cascade membrane technology[J]. Carbohydrate Polymers, 2021, 269: 118329. DOI:10.1016/j.carbpol.2021.118329 |
| [22] |
Hsu WH, Qiu WL, Tsao SM, Tseng AJ, Lu MK, Hua WJ, Cheng HC, Hsu HY, Lin TY. Effects of WSG, a polysaccharide from Ganoderma lucidum, on suppressing cell growth and mobility of lung cancer[J]. International Journal of Biological Macromolecules, 2020, 165: 1604-1613. DOI:10.1016/j.ijbiomac.2020.09.227 |
| [23] |
Ma ZB, Ye C, Deng WW, Xu MM, Wang Q, Liu GQ, Wang F, Liu LM, Xu ZH, Shi GY, et al. Reconstruction and analysis of a genome-scale metabolic model of Ganoderma lucidum for improved extracellular polysaccharide production[J]. Frontiers in Microbiology, 2018, 9: 3076. DOI:10.3389/fmicb.2018.03076 |
| [24] |
Liu GQ, Zhao Y, Wang XL, Zhu CY. Biosynthesis and fermentation control of polysaccharides from Ganoderma lucidum[J]. Mycosystema, 2011, 30(2): 198-205. (in Chinese) 刘高强, 赵艳, 王晓玲, 朱朝阳. 灵芝多糖的生物合成和发酵调控[J]. 菌物学报, 2011, 30(2): 198-205. |
| [25] |
Wang Q. Effect of polysacchairdes composition and the related enzymatic analysis in Ganoderma lucidum submerged culture[D]. Wuxi: Master's Thesis of Jiangnan University, 2013 (in Chinese) 王琼. 灵芝菌丝体培养中多糖组分的变化与相关酶活性分析[D]. 无锡: 江南大学硕士学位论文, 2013. |
| [26] |
Xie CL, Yan SW, Zhang ZM, Gong WB, Zhu ZH, Zhou YJ, Yan L, Hu ZX, Ai LZ, Peng YD. Mapping the metabolic signatures of fermentation broth, mycelium, fruiting body and spores powder from Ganoderma lucidum by untargeted metabolomics[J]. LWT, 2020, 129: 109494. DOI:10.1016/j.lwt.2020.109494 |
| [27] |
Wang Q, Wang F, Xu ZH, Ding ZY. Bioactive mushroom polysaccharides: a review on monosaccharide composition, biosynthesis and regulation[J]. Molecules, 2017, 22(6): 955. DOI:10.3390/molecules22060955 |
| [28] |
Chen SL, Xu J, Liu C, Zhu YJ, Nelson DR, Zhou SG, Li CF, Wang LZ, Guo X, Sun YZ, et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum[J]. Nature Communications, 2012, 3: 913. DOI:10.1038/ncomms1923 |
| [29] |
Li Y, Zhao LT, Gu ZH, Li YR, Shi GY, Ding CY. Heterologous expression and characterization of the key enzymes involved in sugar donor synthesis of polysaccharide in Ganoderma lucidum[J]. Microbiology China, 2019, 46(12): 3233-3247. (in Chinese) 李阳, 赵丽婷, 顾正华, 李由然, 石贵阳, 丁重阳. 灵芝多糖糖供体合成途径中关键酶的异源表达及其酶学性质[J]. 微生物学通报, 2019, 46(12): 3233-3247. |
| [30] |
Hu YR, Li MJ, Wang SL, Yue SN, Shi L, Ren A, Zhao MW. Ganoderma lucidum phosphoglucomutase is required for hyphal growth, polysaccharide production, and cell wall integrity[J]. Applied Microbiology and Biotechnology, 2018, 102(4): 1911-1922. DOI:10.1007/s00253-017-8730-6 |
| [31] |
Li MJ, Chen TX, Gao T, Miao ZG, Jiang AL, Shi L, Ren A, Zhao MW. UDP-glucose pyrophosphorylase influences polysaccharide synthesis, cell wall components, and hyphal branching in Ganoderma lucidum via regulation of the balance between glucose-1-phosphate and UDP-glucose[J]. Fungal Genetics and Biology, 2015, 82: 251-263. DOI:10.1016/j.fgb.2015.07.012 |
| [32] |
Xu JW, Ji SL, Li HJ, Zhou JS, Duan YQ, Dang LZ, Mo MH. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene[J]. Bioprocess and Biosystems Engineering, 2015, 38(2): 399-405. DOI:10.1007/s00449-014-1279-1 |
| [33] |
Tang YJ, Zhong J. Exopolysaccharide biosynthesis and related enzyme activities of the medicinal fungus, Ganoderma lucidum, grown on lactose in a bioreactor[J]. Biotechnology Letters, 2002, 24: 1023-1026. DOI:10.1023/A:1015677313598 |
| [34] |
Ji SL, Liu R, Ren MF, Li HJ, Xu JW. Enhanced production of polysaccharide through the overexpression of homologous uridine diphosphate glucose pyrophosphorylase gene in a submerged culture of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes)[J]. International Journal of Medicinal Mushrooms, 2015, 17(5): 435-442. DOI:10.1615/IntJMedMushrooms.v17.i5.30 |
| [35] |
Zhao LN, Xu JW. Overexpression of GDP-D-mannose pyrophosphorylase (GMP) gene improves the production of polysaccharides in Ganoderma lingzhi[J]. Mycosystema, 2021, 40(5): 1099-1109. (in Chinese) 赵丽娜, 徐军伟. 过表达GDP-D-甘露糖焦磷酸化酶(GMP)基因提高灵芝多糖的生产[J]. 菌物学报, 2021, 40(5): 1099-1109. |
| [36] |
Wang T, Wang YH, Chen C, Ren A, Yu HS, Zhao MW. Effect of the heme oxygenase gene on mycelial growth and polysaccharide synthesis in Ganoderma lucidum[J]. Journal of Basic Microbiology, 2021, 61(3): 253-264. DOI:10.1002/jobm.202000622 |
| [37] |
Li HJ, Zhang DH, Yue TH, Jiang LX, Yu XY, Zhao P, Li T, Xu JW. Improved polysaccharide production in a submerged culture of Ganoderma lucidum by the heterologous expression of Vitreoscilla hemoglobin gene[J]. Journal of Biotechnology, 2016, 217: 132-137. DOI:10.1016/j.jbiotec.2015.11.011 |
| [38] |
Zhang QR, Ding CY, Zhou ZM, Gu ZH, Zhang L, Shi GY. Effects of glucose-1-phosphate on the biosynthesis of Ganoderma lucidum polysaccharide[J]. Chinese Journal of Bioprocess Engineering, 2017, 15(4): 9-14. (in Chinese) 张奇茹, 丁重阳, 周哲敏, 顾正华, 张梁, 石贵阳. 葡萄糖-1-磷酸对灵芝多糖合成的影响[J]. 生物加工过程, 2017, 15(4): 9-14. DOI:10.3969/j.issn.1672-3678.2017.04.002 |
| [39] |
Chen Y, Zhao LT, Gu ZH, Li YR, Shi GY, Ding CY. Effect of glycosyltransferase GL24971 on polysaccharide synthesis in Ganoderma lucidum[J]. Food and Fermentation Industries, 2021, 47(23): 1-7. (in Chinese) 陈云, 赵丽婷, 顾正华, 李由然, 石贵阳, 丁重阳. 糖基转移酶GL24971对灵芝多糖合成的影响[J]. 食品与发酵工业, 2021, 47(23): 1-7. DOI:10.13995/j.cnki.11-1802/ts.027324 |
| [40] |
Feng J, Feng N, Tang QJ, Liu YF, Yang Y, Liu F, Zhang JS, Lin CC. Optimization of Ganoderma lucidum polysaccharides fermentation process for large-scale production[J]. Applied Biochemistry and Biotechnology, 2019, 189(3): 972-986. DOI:10.1007/s12010-019-02968-5 |
| [41] |
Long ZD, Xue Y, Ning ZX, Sun JS, Li JG, Su Z, Liu QB, Xu CP, Yan JK. Production, characterization, and bioactivities of exopolysaccharides from the submerged culture of Ganoderma cantharelloideum M. H. Liu[J]. 3 Biotech, 2021, 11(3): 145. DOI:10.1007/s13205-021-02696-w |
| [42] |
Hsieh C, Tseng MH, Liu CJ. Production of polysaccharides from Ganoderma lucidum (CCRC 36041) under limitations of nutrients[J]. Enzyme and Microbial Technology, 2006, 38(1/2): 109-117. |
| [43] |
Ye SQ, Wu H, Yu YG, Chen N, Li JY. Research for different metal ion in Ganoderma lucidum polysaccharides fermentation[J]. Food Research and Development, 2011, 32(1): 106-108. (in Chinese) 叶盛权, 吴晖, 余以刚, 陈娜, 李金燕. 不同金属离子对灵芝多糖液态发酵的影响[J]. 食品研究与开发, 2011, 32(1): 106-108. |
| [44] |
Yao Q, Gao XX, Gong ZY, Kong M, Ren PF, Liu Y, Han JD, Ren HX. Effect of fatty acid supplementation on mycelium biomass, polysaccharide and triterpene production by Ganoderma lucidum grown in submerged culture[J]. Acta Edulis Fungi, 2010, 17(3): 55-59. (in Chinese) 姚强, 高兴喜, 宫志远, 孔明, 任鹏飞, 刘岩, 韩建东, 任海霞. 不同脂肪酸对灵芝液体发酵的影响[J]. 食用菌学报, 2010, 17(3): 55-59. |
| [45] |
Huang HC, Chen CI, Hung CN, Liu YC. Experimental analysis of the oil addition effect on mycelia and polysaccharide productions in Ganoderma lucidum submerged culture[J]. Bioprocess and Biosystems Engineering, 2009, 32(2): 217-224. DOI:10.1007/s00449-008-0239-z |
| [46] |
Yang HL, Min WH, Bi PY, Zhou HB, Huang FR. Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum[J]. Biochemical Engineering Journal, 2013, 76: 77-82. DOI:10.1016/j.bej.2013.04.012 |
| [47] |
Ma ZB, Xu MM, Wang Q, Wang F, Zheng HH, Gu ZH, Li YR, Shi GY, Ding ZY. Development of an efficient strategy to improve extracellular polysaccharide production of Ganoderma lucidum using L-phenylalanine as an enhancer[J]. Frontiers in Microbiology, 2019, 10: 2306. |
| [48] |
Yang XB, Yang YY, Zhang YF, He JH, Xie YZ. Enhanced exopolysaccharide production in submerged fermentation of Ganoderma lucidum by Tween 80 supplementation[J]. Bioprocess and Biosystems Engineering, 2021, 44(1): 47-56. |
| [49] |
Gao XX, Yao Q, Wang L, Liu LD, Wang WW, Han K. Effect of different fungal elicitors on production of polysaccharide and triterpenoid in liquid fermentation of Ganoderma lucidum[J]. Food Science, 2009, 30(23): 309-313. (in Chinese) 高兴喜, 姚强, 王磊, 刘林德, 王雯雯, 韩凯. 真菌激发子对灵芝液体发酵生产多糖和三萜类物质的影响[J]. 食品科学, 2009, 30(23): 309-313. |
| [50] |
Yao Q, Gao XX, Gong ZY, Ren PF, Qiu KX, Liu Y. Effects of some rare earth elements on liquid fermentation of Ganoderma lucidum to produce polysaccharides and triterpenoids[J]. Food Science, 2011, 32(5): 224-227. (in Chinese) 姚强, 高兴喜, 宫志远, 任鹏飞, 仇可新, 刘岩. 部分稀土元素对灵芝多糖和三萜类物质液体发酵的影响[J]. 食品科学, 2011, 32(5): 224-227. |
| [51] |
Liu GQ, Ding CY, Zhang KC, Wang XL, Han WJ. Effects of medicinal insect, Catharsius molossus on biosynthesis of polysaccharides from Ganoderma lucidum in submerged culture[J]. Chinese Journal of Biotechnology, 2009, 25(6): 914-919. (in Chinese) 刘高强, 丁重阳, 章克昌, 王晓玲, 韩文军. 药用昆虫蜣螂对灵芝多糖生物合成的影响[J]. 生物工程学报, 2009, 25(6): 914-919. |
| [52] |
Zhu Q, Xia YQ, Wang ZJ, Yang CF. Effects of four herbs on growth and fermentation of Ganoderma lucidum[J]. China Brewing, 2010, 29(7): 163-165. (in Chinese) 朱强, 夏艳秋, 汪志君, 杨从发. 4种中药对灵芝生长与发酵的影响[J]. 中国酿造, 2010, 29(7): 163-165. |
| [53] |
Zapata P, Rojas D, Atehortúa L. Production of biomass, polysaccharides, and ganoderic acid using non-conventional carbon sources under submerged culture of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt. : Fr.) P. Karst. (higher Basidiomycetes)[J]. International Journal of Medicinal Mushrooms, 2012, 14(2): 197-203. |
| [54] |
Zhang DX, Wang XL, Liu GQ. Medium optimization for β-glucan production from Ganoderma lingzhi in submerged fermentation[J]. Mycosystema, 2016, 35(6): 750-759. (in Chinese) 张冬雪, 王晓玲, 刘高强. 灵芝菌丝液体深层发酵产β-葡聚糖的培养基优化[J]. 菌物学报, 2016, 35(6): 750-759. |
| [55] |
Peng L, Li J, Liu Y, Xu ZH, Wu JY, Ding ZY, Gu ZH, Zhang L, Shi GY. Effects of mixed carbon sources on galactose and mannose content of exopolysaccharides and related enzyme activities in Ganoderma lucidum[J]. Royal Society of Chemistry Advances, 2016, 6(45): 39284-39291. |
| [56] |
Li J, Ding CY, Gu ZH, Zhang L, Shi GY. Effects of mixed carbon sources on production and antitumor activity of Ganoderma lucidum exopolysaccharides by submerged culture[J]. Journal of Food Science and Biotechnology, 2017, 36(2): 129-135. (in Chinese) 李洁, 丁重阳, 顾正华, 张梁, 石贵阳. 混合碳源对灵芝多糖发酵及其抗肿瘤活性的影响[J]. 食品与生物技术学报, 2017, 36(2): 129-135. |
| [57] |
Qian L, Zhang ZJ, Chen XM, Li SF, Li FM, Liu JH. Optimization of fermentation medium for production of extracellular polysaccharide from Ganoderma lucidum[J]. Food Research and Development, 2017, 38(17): 10-13. (in Chinese) 钱磊, 张志军, 陈晓明, 李淑芳, 李凤美, 刘建华. 灵芝液体发酵产胞外多糖培养基的优化[J]. 食品研究与开发, 2017, 38(17): 10-13. |
| [58] |
Fraga I, Coutinho J, Bezerra RM, Dias AA, Marques G, Nunes FM. Influence of culture medium growth variables on Ganoderma lucidum exopolysaccharides structural features[J]. Carbohydrate Polymers, 2014, 111: 936-946. |
| [59] |
Zeng XX, Zhou HB, Fu B, Liu XD. Study on fermentation conditions of Ganoderma lucidum[J]. Progress in Modern Biomedicine, 2007, 7(6): 830-832. (in Chinese) 曾晓希, 周洪波, 符波, 刘学端. 灵芝多糖液体发酵条件的研究[J]. 现代生物医学进展, 2007, 7(6): 830-832. |
| [60] |
Xue FF, Lei HJ, Zhang YH, Wang XL, He HJ, Liu GQ. Effects of some exogenous substances on polysaccharide and β-glucan syntheses of Ganoderma lingzhi in submerged fermentation[J]. Mycosystema, 2020, 39(1): 164-173. (in Chinese) 薛菲菲, 雷含珺, 张漾泓, 王晓玲, 何含杰, 刘高强. 外源物质对灵芝多糖和β-葡聚糖发酵的影响[J]. 菌物学报, 2020, 39(1): 164-173. |
| [61] |
Kim HM, Park MK, Yun JW. Culture pH affects exopolysaccharide production in submerged mycelial culture of Ganoderma lucidum[J]. Applied Biochemistry and Biotechnology, 2006, 134(3): 249-262. |
| [62] |
Qiao SK, Peng L, Ding CY, Gu ZH, Zhang L, Shi GY. Effect of different culture conditions on mycelium morphology and activity of exopolysaccharides from Ganoderma lucidum in submerged culture[J]. Journal of Food Science and Biotechnology, 2014, 33(10): 1070-1076. (in Chinese) 乔双逵, 彭林, 丁重阳, 顾正华, 张梁, 石贵阳. 液体发酵条件对灵芝菌体形态及胞外多糖活性的影响[J]. 食品与生物技术学报, 2014, 33(10): 1070-1076. |
| [63] |
Tang YJ, Zhong JJ. Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of Ganoderma polysaccharide and ganoderic acid[J]. Enzyme and Microbial Technology, 2003, 32(3/4): 478-484. |
| [64] |
Kim HM, Paik SY, Ra KS, Koo KB, Yun JW, Choi JW. Enhanced production of exopolysaccharides by fed-batch culture of Ganoderma resinaceum DG-6556[J]. Journal of Microbiology, 2006, 44(2): 233-242. |
| [65] |
Fazenda ML, Harvey LM, McNeil B. Effects of dissolved oxygen on fungal morphology and process rheology during fed-batch processing of Ganoderma lucidum[J]. Journal of Microbiology and Biotechnology, 2010, 20(4): 844-851. |
| [66] |
Tang YJ, Zhang W, Liu RS, Zhu LW, Zhong JJ. Scale-up study on the fed-batch fermentation of Ganoderma lucidum for the hyperproduction of ganoderic acid and Ganoderma polysaccharides[J]. Process Biochemistry, 2011, 46(1): 404-408. |
| [67] |
Zhou XP, Yang HJ. Effects of dissolved oxygen on exocellular polysaccharides produced by deep liquid cultivation of Ganoderma lucidum[J]. Hubei Agricultural Sciences, 2011, 50(18): 3804-3807. (in Chinese) 周小苹, 杨海军. 溶氧对灵芝液体深层培养产胞外多糖的影响[J]. 湖北农业科学, 2011, 50(18): 3804-3807. |
| [68] |
Wu D, Yuan F, Wang GR, Zhou S, Yang Y, Feng N, Tang QJ, Liu YF, Zhu ZL, Xu GH, et al. A aeration volume strategy for enhanced intracellular polysaccharide production by Ganoderma lucidum mycelium based on submerged culture[J]. Acta Edulis Fungi, 2019, 26(1): 35-40. (in Chinese) 吴迪, 袁峰, 王国瑞, 周帅, 杨焱, 冯娜, 唐庆九, 刘艳芳, 朱增亮, 徐国华, 等. 基于通气量调控的灵芝菌丝体胞内多糖发酵工艺优化[J]. 食用菌学报, 2019, 26(1): 35-40. |
| [69] |
Zhang YN, Qian L, Liu YH, Kong QX, Zhang ZJ. Effect of light on exopolysaccharide synthesis in liquid fermentation of Ganoderma lucidum[J]. Northern Horticulture, 2019(2): 154-159. (in Chinese) 张业尼, 钱磊, 刘逸寒, 孔庆学, 张志军. 光照对灵芝液体发酵胞外多糖合成的影响[J]. 北方园艺, 2019(2): 154-159. |
| [70] |
Liu SR, Zhang WR, Kuang YB, Zheng SY. Sonoporation of Ganoderma lucidum mycelium for high biomass and exopolysaccharide productivity in submerged culture[J]. International Journal of Agriculture and Biology, 2016, 18(4): 773-779. |
| [71] |
Sun L, Liu LP, Yang L, Wang YZ, Dabbour M, Mintah BK, He RH, Ma HL. Effects of low-intensity ultrasound on the biomass and metabolite of Ganoderma lucidum in liquid fermentation[J]. Journal of Food Process Engineering, 2021, 44(1): e13601. |
| [72] |
Zhu LW, Zhong JJ, Tang YJ. Multi-fed batch culture integrated with three-stage light irradiation and multiple additions of copper ions for the hyperproduction of ganoderic acid and Ganoderma polysaccharides by the medicinal mushroom Ganoderma lucidum[J]. Process Biochemistry, 2010, 45(12): 1904-1911. |
| [73] |
Balamurugan JP, Supramani S, Ahmad Usuldin SR, Ilham Z, Klaus A, Khairul Ikram NK, Ahmad R, Wan Abd Al Qadr Imad Wan-Mohtar. Efficient biomass- endopolysaccharide production from an identified wild-Serbian Ganoderma applanatum strain BGS6Ap mycelium in a controlled submerged fermentation[J]. Biocatalysis and Agricultural Biotechnology, 2021, 37: 102166. |
| [74] |
Lee WY, Park Y, Ahn JK, Ka KH, Park SY. Factors influencing the production of endopolysaccharide and exopolysaccharide from Ganoderma applanatum[J]. Enzyme and Microbial Technology, 2007, 40(2): 249-254. |
| [75] |
Liu YQ, He XL, Zhao RH, Ren GM. Effects of increasing liquid fermentation temperature of Ganoderma lucidum on its active component content[J]. Northern Horticulture, 2020(15): 118-124. (in Chinese) 刘月芹, 贺晓龙, 赵瑞华, 任桂梅. 提高灵芝液体发酵温度对其活性成分含量的影响[J]. 北方园艺, 2020(15): 118-124. |
| [76] |
Liu YQ, He XL, Ren GM. Effect of higher temperature on culture properties of Ganoderma lucidum and ganoderic acid yield[J]. Journal of Microbiology, 2019, 39(2): 90-96. (in Chinese) 刘月芹, 贺晓龙, 任桂梅. 较高温度对灵芝培养特性及灵芝酸产量的影响[J]. 微生物学杂志, 2019, 39(2): 90-96. |
| [77] |
Peng L, Qiao SK, Xu ZH, Guan F, Ding ZY, Gu ZH, Zhang L, Shi GY. Effects of culture conditions on monosaccharide composition of Ganoderma lucidum exopolysaccharide and on activities of related enzymes[J]. Carbohydrate Polymers, 2015, 133: 104-109. |
| [78] |
Zapata PA, Rojas DF, Ramírez DA, Fernández C, Atehortúa L. Effect of different light-emitting diodes on mycelial biomass production of ling Zhi or Reishi medicinal mushroom Ganoderma lucidum (W. Curt. : Fr.) P. karst. (Aphyllophoromycetideae)[J]. International Journal of Medicinal Mushrooms, 2009, 11(1): 93-99. |
| [79] |
Zhang W, Tang YJ. A novel three-stage light irradiation strategy in the submerged fermentation of medicinal mushroom Ganoderma lucidum for the efficient production of ganoderic acid and Ganoderma polysaccharides[J]. Biotechnology Progress, 2008, 24(6): 1249-1261. |
| [80] |
Yuwu MX, Lan J, Zhang WW, Xu XR, Liu Y, Yin CM, Yu J, Chen XD. Effects of blue light on polysaccharides accumulation and glycometabolism-related enzymes of Ganoderma lucidum mycelium[J]. Chinese Agricultural Science Bulletin, 2017, 33(36): 47-51. (in Chinese) 余吴梦晓, 兰进, 张薇薇, 徐新然, 刘禹, 尹春梅, 于晶, 陈向东. 蓝光对灵芝菌丝体多糖积累和糖代谢相关酶的影响[J]. 中国农学通报, 2017, 33(36): 47-51. |
| [81] |
Fang QH, Zhong JJ. Effect of initial pH on production of ganoderic acid and polysaccharide by submerged fermentation of Ganoderma lucidum[J]. Process Biochemistry, 2002, 37(7): 769-774. |
| [82] |
Zhang XH, Hu HB, Tang YL, Huang RS, Chen ZJ. Continuous fermentation of Ganoderma lucidum exo-polysaccharide in a membrane bioreactor[J]. Journal of Chemical Engineering of Chinese Universities, 2002, 16(6): 670-674. (in Chinese) 张雪洪, 胡洪波, 唐涌濂, 黄瑞珊, 陈正杰. 膜生物反应器连续发酵生产胞外灵芝多糖[J]. 高校化学工程学报, 2002, 16(6): 670-674. |
| [83] |
Wan Abd Al Qadr Imad Wan Mohtar, Ab Latif N, Harvey LM, McNeil B. Production of exopolysaccharide by Ganoderma lucidum in a repeated-batch fermentation[J]. Biocatalysis and Agricultural Biotechnology, 2016, 6: 91-101. |
| [84] |
Sun L, Liu LP, Wang YZ, Yang L, Zhang CS, Yue MX, Dabbour M, Mintah BK, Wang L. Effect of ultrasonication on the metabolome and transcriptome profile changes in the fermentation of Ganoderma lucidum[J]. Microbiological Research, 2022, 254: 126916. |
| [85] |
Ahmad R, Lim CK, Marzuki NF, Goh YK, Azizan KA, Goh YK, Goh KJ, Ramzi AB, Baharum SN. Metabolic profile of Scytalidium parasiticum-Ganoderma boninense co-cultures revealed the alkaloids, flavonoids and fatty acids that contribute to anti-Ganoderma activity[J]. Molecules, 2020, 25(24): 5965. |
| [86] |
Asadi F, Barshan-tashnizi M, Hatamian-Zarmi A, Davoodi-Dehaghani F, Ebrahimi-Hosseinzadeh B. Enhancement of exopolysaccharide production from Ganoderma lucidum using a novel submerged volatile co-culture system[J]. Fungal Biology, 2021, 125(1): 25-31. |
| [87] |
Wu JY, Kaewnarin K, Nie XM, Li QB, He N, Huang JL, Geng AL. Biological activities of a polysaccharide from the coculture of Ganoderma lucidum and Flammulina velutipes mycelia in submerged fermentation[J]. Process Biochemistry, 2021, 109: 10-18. |
| [88] |
Yao L, Zhu LP, Xu XY, Tan LL, Sadilek M, Fan H, Hu B, Shen XT, Yang J, Qiao B, et al. Discovery of novel xylosides in co-culture of Basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics[J]. Scientific Reports, 2016, 6: 33237. |
 2022, Vol. 49
2022, Vol. 49