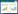扩展功能
文章信息
- 贾文馨, 王丽蕊
- JIA Wenxin, WANG Lirui
- 肠道菌群来源细胞外囊泡在肝脏疾病中的作用研究进展
- Research progress in the relationship between gut microbiota-derived extracellular vesicles and liver diseases
- 微生物学通报, 2023, 50(9): 4206-4219
- Microbiology China, 2023, 50(9): 4206-4219
- DOI: 10.13344/j.microbiol.china.230262
-
文章历史
- 收稿日期: 2023-03-31
- 接受日期: 2023-05-22
- 网络首发日期: 2023-06-16
2. 南京大学现代生物研究院, 江苏 南京 210008
2. Institute of Modern Biology, Nanjing University, Nanjing 210008, Jiangsu, China
细胞外囊泡(extracellular vesicles, EVs)是一类由所有活细胞在不同产生途径下分泌的具有脂质双分子层的球形囊泡[1-2],可以携带多种类型的分子,如蛋白质、RNA、DNA和脂质[3]。自Pan等首次观察到网织红细胞可释放细胞外囊泡以来[4],后续研究表明它们存在于几乎所有类型的哺乳动物细胞,包括各种癌细胞系[5]。1976年,Hoekstra等[6]在电子显微镜下观察到大肠杆菌(Escherichia coli)可以释放囊泡。之后,大部分革兰氏阴性菌和革兰氏阳性菌被证实可以分泌直径为20−300 nm的囊泡[7]。根据细菌分泌囊泡的结构和组成差异,人们将细菌来源的EVs分为革兰氏阴性菌的细菌外膜囊泡(outer membrane vesicles, OMVs)和外-内膜囊泡(outer-intimal membrane vesicles, OIMVs),以及革兰氏阳性菌的膜囊泡(membrane vesicles, MVs)[8]。除细菌外,也有研究发现真菌可以产生囊泡,并且真菌可以通过囊泡传递信息参与维持调控机体的稳态[9-10]。
人体的皮肤和各种黏膜表面定居的大量微生物统称为微生物组[11]。其中肠道微生物群被认为是一个“重要器官”,结肠中每克湿重粪便中的微生物数量超过1011个,它所包含的基因组大约是目前已发现人类基因组的150倍,远远超过并补充了人类基因组编码的遗传信息[12-13]。由于微生物进入肠上皮受到物理和化学上的限制,微生物与宿主的交流主要依赖于微生物分泌的因子,如代谢物、蛋白质和EVs,它们可以穿过黏蛋白层被肠黏膜表面的宿主细胞内化。越来越多的证据表明,细菌分泌的装载着具有生物活性分子的囊泡被宿主细胞识别并整合入细胞内,能调控宿主细胞的信号通路,调节宿主的生理和病理过程[14-15]。肠道和肝脏在解剖学上接近,肝脏与肠道通过胆道、肝门静脉等通路进行双向交流。肠道菌群及其分泌的代谢物或囊泡与肝脏之间的信息通讯对维持宿主健康至关重要[16-18]。在酒精或者脂毒性影响下,肠道微生态系统发生紊乱,表现为菌群多样性改变,肠屏障破坏,并且肠道微生物及其代谢产物会通过被破坏的肠屏障易位至肝脏中,激活先天免疫系统受体并诱导肝脏脂质合成增加,促进肝脏疾病的发展。近年来,探究细胞外囊泡在介导慢性肝病发病中的作用以及作为慢性肝病的非侵入性诊断工具是国内外研究热点[19-21]。然而,目前研究大多集中在肝脏自身分泌的细胞外囊泡在肝脏疾病中的作用[22],对于肠道来源的细胞外囊泡,尤其是微生物来源的细胞外囊泡在肝脏疾病中的调控作用及其背后的分子机制鲜有报道。本文关注细菌来源细胞外囊泡的产生过程、内容物及其在细菌-宿主互作的方式,最后探讨肠道菌群来源的细胞外囊泡在肝脏疾病中的作用。
1 细菌来源的细胞外囊泡:生物产生在真核生物中,根据产生机制的不同,EVs被分为3种类型:外泌体(exosomes)、微囊泡(microvesicles)和凋亡小体[23]。外泌体是直径为50−150 nm膜形囊泡,其产生过程涉及细胞质膜双重内陷和含腔内小泡(intraluminal vesicles, ILVs)的多泡内体(multivesicular bodies, MVBs)的形成[1, 24-25]。与外泌体不同,微囊泡的产生不经过多泡内体过程,而是直接从质膜上出芽,释放一类直径为0.1−1.0 μm的囊泡;凋亡小体是在细胞发生凋亡时细胞骨架破裂和分解细胞碎片过程中释放的EVs,其直径约50−5 000 nm (图 1)[3]。
|
| 图 1 真核细胞细胞外囊泡产生过程 Figure 1 Generation and release of extracellular vesicle in eukaryotic cells. 外泌体早期生物合成过程中,细胞外成分如蛋白质与细胞膜表面蛋白随细胞膜内陷所包裹起来,形成一个被称为早期分选核内体(early sorting endosome, ESE)的杯状结构. 随后,ESE在内质网、高尔基体的作用下逐渐成熟成为晚期分选核内体(late sorting endosome, LSE),LSE膜内陷形成多个腔内小泡(intraluminal vesicles, ILVs),最终形成多泡内体(multivesicular body, MVB). 细胞质膜和MVB相互融合之后,ILVs就成为直径50−150 nm的外泌体被释放到细胞外,在外泌体形成过程中有细胞骨架蛋白如actin参与. 微囊泡则是在骨架蛋白myosin-Ⅱ和cortical actin作用下直接从质膜上出芽,被释放到胞外 During the biosynthesis of exosomes, extracellular components such as proteins and cell membrane surface proteins are encapsulated by cell membrane invaginations, forming a cup-shaped structure called early sorting endosome (ESE). Subsequently, ESE gradually matures into late sorting endosomes (LSEs) under the action of endoplasmic reticulum and Golgi apparatus, and LSEs membrane invaginates to form multiple intraluminal vesicles (ILVs), and finally multivesicular bodies (MVBs). After the plasma membrane and MVBs fuse with each other, ILVs become exosomes with a diameter of 50−150 nm and are released outside the cell. Cytoskeletal proteins such as actin are involved in the formation of exosomes. Microvesicles directly bud from the plasma membrane under the action of the skeleton protein myosin-Ⅱ and cortical actin, and are released to the outside of the cell. |
|
|
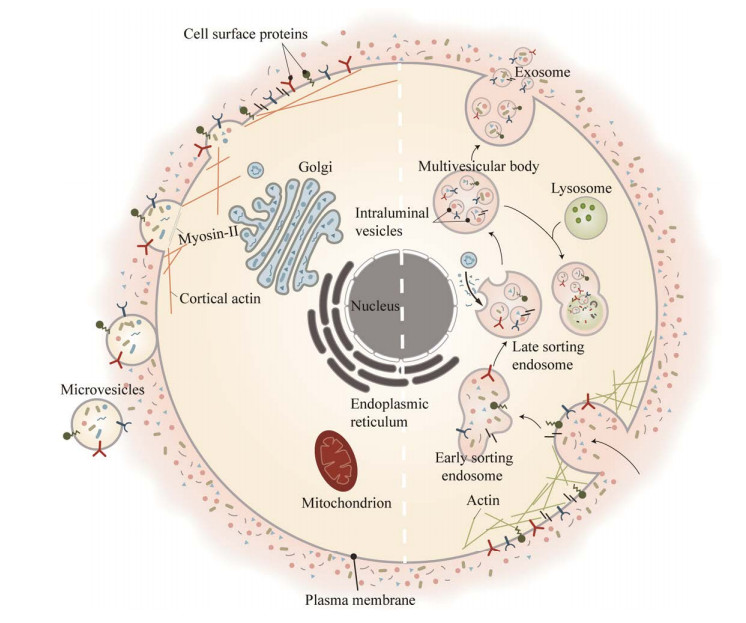
细菌正常生长和应激时可以释放直径为20‒400 nm不等的膜囊泡,影响多种生物过程包括细胞间通信和噬菌体感染[26-27],革兰氏阴性菌来源的OMVs主要通过3种模型产生[7, 26]:(1) 与膜交联调节相关(图 2A)。在细胞壁循环过程中,肽聚糖层(peptidoglycan, PG)和外膜之间的交联被破坏导致外膜出芽,OMVs被释放到胞外空间[6, 28-29]。相反地,若增加脂蛋白-PG交联则会使得OMVs分泌减少[30]。(2) 与脂质和脂质结合分子作用相关(图 2B)。喹诺酮假单胞菌信号(pseudomonas quinolone signal, PQS)中2-烷基和3-羟基与脂质A相互作用,刺激革兰氏阴性菌细胞膜出芽,从而细菌分泌OMVs增加[31-33]。(3) 与膜曲率诱导分子的聚集相关(图 2C)。肽聚糖碎片或错误折叠的蛋白质大量积累在周质(periplasm),导致膨胀压力增加从而使得外膜膨出,OMVs被释放到胞外[34-35]。另外,脂多糖(lipopolysaccharide, LPS)增加可以刺激细菌产生更多的OMVs,其主要原因是LPS分子之间出现阴离子排斥,随后细菌细胞膜出现局部变形而分泌囊泡[33, 36-37]。OMVs的产生受多种因素的影响,其生物发生机制有待进一步研究。
|
| 图 2 原核细胞中不同的细胞外囊泡产生过程 Figure 2 Different extracellular vesicle production processes in prokaryotic cells. A、B、C为革兰氏阴性菌产生细菌外膜囊泡(outer membrane vesicles, OMVs)的3种模型. A:革兰氏阴性菌外膜的脂蛋白(lipoprotein, Lpp)和肽聚糖层(peptidoglycan, PG)之间形成交联的能力受到肽聚糖内肽酶或其他因素影响,在低Lpp-PG交联区域,膜流动性增加从而使得OMVs产生增加. B:喹诺酮假单胞菌信号(pseudomonas quinolone signal, PQS)会与外膜的脂质A相互作用,增加膜曲率,导致OMVs的形成. C:错误折叠的蛋白质或包膜成分如脂多糖(lipopolysaccharide, LPS)或PG碎片所堆积的区域,使得交联被破坏膨胀压力增加,促进这外膜区域膨胀,导致OMVs产生增加. D:内溶菌素能降解革兰氏阳性菌厚的肽聚糖壁并使细胞壁上出现小孔,继而导致细胞质膜从这些小孔中突出,并自发转化为细菌细胞外囊泡,引发“气泡细胞死亡”,但其潜在机制尚不清楚 A, B and C are three models of bacterial outer membrane vesicles (OMVs) produced by Gram-negative bacteria. A: Lipoprotein (Lpp) and the ability to form crosslinks between peptidoglycan (PG) is affected by peptidoglycan endopeptidases or other factors. In regions of low Lpp-PG crosslinking, increased membrane fluidity leads to increased OMVs production. B: Pseudomonas quinolone signal (PQS) interacts with lipid A of the outer leaflet of the outer membrane, increasing membrane curvature, resulting in Formation of OMVs. C: Areas where misfolded proteins or envelope components such as lipopolysaccharide (LPS) or PG fragments accumulate, causing cross-links to be destroyed and swelling pressure increased, which promotes the expansion of this outer membrane area, resulting in increased OMVs production. D: Endolysins degrade the thick peptidoglycan wall of Gram-positive bacteria and create pores in the cell wall, which in turn cause the plasma membrane to protrude from these pores and spontaneously transform into bacterial EVs, triggering "bubble cell death", but the underlying mechanism remains unclear. |
|
|
相较于革兰氏阴性菌,对革兰氏阳性菌产生的MVs研究甚少。Lee等在2009年研究中首次从金黄色葡萄球菌和枯草芽孢杆菌的培养上清中分离出革兰氏阳性细胞外囊泡[38]。革兰氏阳性菌含有内溶菌素,它是一种能降解肽聚糖的酶,能降解革兰氏阳性菌厚的肽聚糖壁并使细胞壁上出现小孔,继而导致细胞质膜从这些小孔中突出,并自发转化为细菌MVs (图 2D)[8]。
2 细菌来源的细胞外囊泡:组成成分EVs具有囊形结构,可以携带不同类型的功能分子如蛋白质(膜蛋白、细胞质蛋白和核蛋白、细胞外基质蛋白)、RNA (mRNA、miRNA、IncRNA和其他RNA)、DNA (mtDNA、ssDNA和dsDNA)和脂质[39-41],这些分子可以反映囊泡的起源及其供体细胞所处的病理生理状态[42]。
细菌分泌的EVs内容物主要包含三大类:蛋白质、脂质和核酸,有研究表明其中成分还会受环境因素,如温度和pH的影响而改变[26]。革兰氏阴性菌来源的OMVs中的脂质和蛋白质分布与供体外膜非常相似,其中OMVs的磷脂存在于膜内侧,LPS存在于膜外侧,并与外膜蛋白和脂蛋白混合[43-44]。但OMVs的内容物含量与成分和供体细胞并不完全相同[45-47],如OMVs中富集一些外膜蛋白包括OmpA和AcrA等周质蛋白。此外,研究者还发现OMVs中存在毒力因子和大量具有致病性的蛋白质[48-50]。
目前对革兰氏阳性菌来源MVs的内容物知之甚少。有2项脂质组学分析显示,革兰氏阳性细菌的MVs由多种脂肪酸组成,炭疽杆菌和肺炎链球菌来源的MVs富含肉豆蔻酸等短链饱和脂肪酸(C12‒C16)[51-52]。Clostridium perfringens产生的MVs中含有16S核糖体RNA、编码α毒素的DNA[31]。再者,有研究对金黄色葡萄球菌MVs进行蛋白质组学分析,鉴定出了165种蛋白质,其中富含细胞外膜的毒力相关蛋白,包括IgG结合蛋白SbI和β-内酰胺酶,这些蛋白广泛参与了细菌-细菌和细菌-宿主相互作用的过程[38]。
如表 1所示,不同来源的EVs内容物主要包括蛋白质、脂质和核酸三大类,但具体成分不尽相同,它们分布于EVs的膜上或腔内且发挥着不同的功能作用。这些功能分子可表征EVs的来源,如CD9和TSG101是真核生物来源EVs的生物标志物[53]。
| Type | Position | Composition | Function | References |
| Exosomes | Member | Tetraspanins: CD9, CD81, CD63, CD53 | Membrane organisers | [53] |
| Annexin V, lactadherin | Cell adhesion | [3] | ||
| RAB, GTPases, annexins | Intracellular trafficking | [54] | ||
| A33, EpCAM, CD11c | Cell-type-specific proteins | [55-56] | ||
| Lumen | Elongation factors, GAPDH | Enzymes | [3] | |
| Protein kinases, G proteins | Signal transduction | [3] | ||
| ALIX, TSG101, syntenin-1 | Biogenesis factors | [53] | ||
| HSP70, HSP90 | Chaperones | [57] | ||
| microRNA and other non-coding RNAs, mRNA, DNA | Nucleic acids | [58] | ||
| Outer membrane vesicles | Member | β-lactamase, OprF, PonA | Antibiotic resistance | [50, 59] |
| LPS, PQS, peptidoglycan | Biogenesis factors | [59] | ||
| Adhesin/Lnvasin, OmpA, OmpC, OmpF | Bacteria adhesion and invasion | [43] | ||
| Cytolysin A, protease cholera toxin, shiga toxin | Host cell modulation | [60] | ||
| EstA, FlgE, OprF, OprG | Host-bacteria interaction | [50] | ||
| Lumen | Periplasmic proteins alkaline phosphatase, AcrA | Virulence factor delivery | [48] | |
| Endopeptidase L5, peptidoglycan hydrolase | Enzymes | [8] | ||
| DNA, degP | Biofilm formation | [59] | ||
| Membrane vesicles | Member | Lipoteichoic acid (LTA) | [61] | |
| β-lactamase, penicillin-binding proteins: PBP1 | Antibiotic resistance | [62-63] | ||
| InlB, lgG-binding protein Sbl, protective antigen, lethal factor | Virulence factor Delivery | [38] | ||
| Plasma-binding proteins, staphopain A | Bacteria adhesion and lnvasion | [38] | ||
| α-hemolysin, proteolysin, β2 toxin, superantigens: SSaA1, SSaA2 | Host cell modulation | [38] | ||
| Lumen | DNA, RNA (including mRNA, rRNA, sRNA, and tRNA) | Bacterial communities | [60] | |
| Lipoprotein (MalX), transmembrane Protein (PspA), pneumolysin | Toxin | [52] | ||
| Dehydrogenase, DNA polymerases, tRNA synthetases | Metabolism associated | [31, 38] | ||
| GAPDH:甘油醛-3-磷酸脱氢酶;ALIX:ALG-2相互作用蛋白X;TSG101:肿瘤易感基因101蛋白;RAB:RAS-相关蛋白;LPS:脂多糖;PQS:喹诺酮类假单胞菌信号;OmpA:外膜蛋白A GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; ALIX: ALG-2 interacting protein X; TSG101: Tumour susceptibility gene 101 protein; RAB: RAS-related protein; LPS: Lipopolysaccharides; PQS: Pseudomonas quinolone signal; OmpA: Outer membrane protein A. | ||||
细胞可以通过分泌EVs与邻近或远处细胞进行通信,细菌通过分泌EVs直接激活靶细胞或将EVs内的物质转移到受体细胞,参与细菌-细菌和细菌-宿主相互作用。
细菌来源的EVs参与细菌间互作有3种方式:群体感应、生物膜形成和相互竞争。据报道,铜绿假单胞菌(Pseudomonas aeruginosa)利用其EVs运输群体感应分子PQS,PQS结合LPS直接与细菌相互作用[32];另外,变形链球菌(Streptococcus mutans)是人类龋齿的主要病原体,在细菌外膜产生过程中会大量释放含有细胞外DNA的MVs,这些囊泡相关的细胞外DNA有助于生物膜的形成,并影响生物膜的结构完整性和稳定性,这可能是由于细胞外DNA与细胞外聚合物(extracellular polymeric substance)中的其他成分如多糖特异性结合导致;此外,EVs可作为竞争武器,如铜绿假单胞菌EVs含有的水解酶可降解肽聚糖竞争性杀死其他细菌[64-66]。
细菌来源的EVs参与细菌-宿主相互作用的方式有2种:(1) EVs被宿主细胞内化,从而内容物被传递到宿主细胞细胞质中。宿主细胞对细菌EVs的摄取主要通过内吞作用,这一过程的机制取决于囊泡的膜表面蛋白和内容物。最近的一项报道揭示了宿主细胞对常驻肠道菌多形拟杆菌(Bacteroides thetaiotaomicron)的OMVs摄取和运输机制[67]。研究者发现B. thetaiotaomicron来源的OMVs主要通过动力依赖的内吞作用被肠上皮细胞内化,并被运输到溶酶体内的囊泡;此外,体内成像研究表明,部分B. thetaotaomicron OMVs通过细胞旁转运穿过肠上皮并到达肝脏,这表明细菌来源的EVs可介导与肠外组织的远距离通信[67]。被宿主细胞内吞后,EVs所携带的sRNA、DNA、毒力因子诱导细胞炎症因子分泌,引发宿主炎症反应或细胞毒性反应[68-69]。(2) EVs被宿主细胞膜表面受体识别。细菌来源EVs含有供体产生的生物活性成分,如病原体相关分子模式(pathogen-associated molecular patterns, PAMPs)可被宿主上皮细胞和免疫细胞表达的模式识别受体(pattern recognition receptors, PRRs)识别;Toll样受体(Toll-like receptor, TLR)作为一种PRRs,通常位于细胞质膜,可以与细菌来源EVs膜表面的LPS和脂蛋白结合,从而激活并参与调节免疫和防御反应的细胞信号通路[70-71]。此外,细胞质中还存在免疫受体,如核苷酸结合寡聚结构域蛋白1 (nucleotide-binding oligomerization domain protein 1, NOD1)和NOD2。革兰氏阴性菌侵染宿主时,OMVs在细胞内运输过程中NOD1被招募,NOD1与内吞囊泡中包含的肽聚糖相互作用[72]。结合肽聚糖后,这些受体通过激活NF-κB或丝裂原激活蛋白激酶途径启动炎症反应,最终上调炎症基因[72]。
4 肠道菌群来源细胞外囊泡在肝脏疾病中的作用鉴于肝脏和肠道在解剖和功能上存在密切联系,肠腔中的囊泡极有可能会通过肠-肝轴进入肝脏,从而影响肝脏生理过程。有研究人员构建了表达Cre酶的工程大肠杆菌(E. coliCre),并定殖在Rosa26.tdTomato背景的小鼠中,导致Cre酶诱导的荧光报告基因在肠道上皮细胞中表达,包括肠道干细胞以及巨噬细胞等黏膜免疫细胞;在肠道外,OMVs可越过肠道屏障转移到各种宿主组织中,包括心脏、肝脏、肾脏和大脑[73]。研究表明肝脏是EVs摄取的活跃部位[74],OMVs主要集中在肝小叶汇管区,该区为肝动脉、肝门静脉和小胆管交汇处[73]。
近年来,随着对EVs的深入研究,细菌来源的EVs可作用于肝脏中各类型细胞,其在非酒精性脂肪性肝病、肝癌在内的各种肝脏疾病中的作用也被逐步发掘(图 3)。非酒精性脂肪性肝炎病人在循环血中细菌细胞外囊泡含量相较于健康人含量增多,而且粪便中的细胞外囊泡可通过抑制非肌球蛋白轻链激酶(nonmuscular myosin light chain kinase, nmMLCK)蛋白进而抑制肠上皮细胞紧密连接蛋白,如ZO-1的表达,增加肠道通透性,细胞外囊泡到达肝脏后增加肝脏内血管通透性以及肝脏内皮细胞炎症细胞因子和趋化因子的水平,激活PS/TLR4通路,从而影响了NASH的发生发展[75]。高脂饮食小鼠或二型糖尿病人粪便中的EVs富含磷脂酰胆碱(phosphatidylcholine, PC),PC可以结合并激活肝脏中的芳香烃受体(aryl hydrocarbon receptor, AHR),使得胰岛素信号通路相关基因包括IRS-2及其下游基因PI3K和Akt的表达受到抑制,从而引起胰岛素抵抗[76]。同时高脂饮食小鼠粪便中的EVs富含细菌DNA,细菌DNA可以通过激活cGAS/STING引发肝细胞炎症和肝星状细胞(hepatic stellate cell, HSC)纤维化激活,这说明微生物DNA可能是肠道细胞外囊泡产生效应的关键货物[77-78]。近年来,幽门螺杆菌感染被认为在一些胃肠外的疾病特别是肝脏疾病中起着重要作用。OMVs是幽门螺杆菌最重要的毒力因子之一。幽门螺杆菌来源的OMVs可能有助于肝实质细胞外泌体膜表面蛋白修饰,修饰后的外泌体可能在HSC激活和肝纤维化进展中发挥作用[79]。然而嗜黏蛋白阿克曼菌(Akkermansia muciniphila)被认为是新一代肠道益生菌,研究表明,A. muciniphila来源的EVs能够改善高脂饮食合并肝纤维化小鼠模型的肠道通透性,同时,A. muciniphila来源的EVs可减少肠道中的病原体,从而调节肝脏炎症反应,缓解肝纤维化,进而可预防肝损伤[80]。目前大部分的研究还停留在体外试验研究,更加深入的研究还有待探讨。
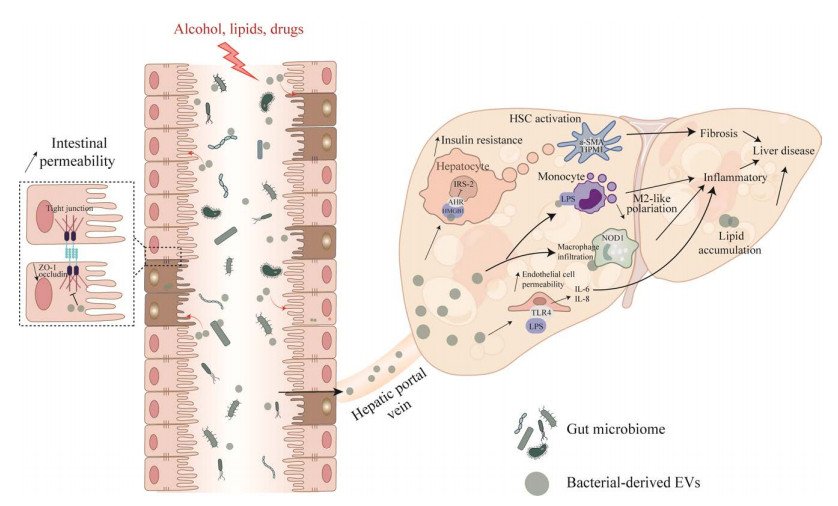
|
| 图 3 肠道菌群来源的细胞外囊泡在肝脏疾病的作用 Figure 3 The role of gut microbiota-derived extracellular vesicles in liver disease. 酒精、各种脂毒性或者药物损伤刺激肠道细菌分泌细胞外囊泡,这些EVs可以抑制肠上皮细胞紧密连接蛋白如ZO-1的表达,增加肠道通透性. EVs通过肠屏障及肝门静脉进入肝脏. 释放的EVs富含各种分子(miRNAs、蛋白质和DNA),并对肝脏中的几种肝细胞类型产生影响. 它们可以激活肝星状细胞并促进纤维化沉积. 它们被单核细胞、巨噬细胞和肝脏内皮细胞吸收,总体上导致肝脏炎症增加. 重要的是肝脏脂质积累也可以由肠道菌群衍生EVs引起 Alcohol, various lipotoxicity, or drug damage stimulate intestinal bacteria to secrete extracellular vesicles (EVs), which can inhibit the expression of intestinal epithelial cell tight junction proteins such as ZO-1 and increase intestinal permeability. EVs pass through the highly permeable intestinal lumen and enter the liver through the hepatic portal vein. The released EVs are enriched in various molecules (miRNAs, proteins, DNA) and exert effects on several hepatocyte types in the liver. They can activate hepatic stellate cells and promote fibrotic deposition. They are taken up by monocytes, macrophages, and liver endothelial cells, resulting in increased liver inflammation overall. Importantly, hepatic lipid accumulation can also be induced by gut-derived EVs. |
|
|
肠道中含有庞大的微生物体系,并且肠道微生物稳态对维持人体健康具有重要意义。然而,关于微生物群作用的内在机制仍亟需探索,尤其是微生物如何将信息传递到靶细胞的有关机制,这对于了解疾病和将微生物群或微生物群衍生的EVs转化至临床有着重要意义。越来越多的证据表明,与疾病相关的微生物组变化可能反映在生物体液中细菌来源的EVs水平和组成上。因此,生物体液中特异性细菌来源的EVs可能与体内特定感染状态有关[81-82]。宏基因组学和代谢组学试验研究已经表明,细菌来源的EVs与阿尔兹海默病[83]、卵巢癌[82]和呼吸系统疾病[84-85]等疾病之间存在着紧密的关联。目前有临床研究发现,在肝脏疾病患者的粪便样本中发现肠道微生物EVs[75],并且EVs数量或者内容物含量与疾病损伤程度呈相关性。在这种情况下,肠道微生物EVs被认为是一种具有潜在价值的疾病诊断工具,主要优势在于:(1) 取样方便且无创,可以实现对患者疾病进程的实时监控;(2) EVs携带特定的蛋白质、核酸等能提供丰富的生物学信息,特异性较高[1];粪便中的EVs在不同的储存条件下具有较好的稳定性,能在−80 ℃长时间保存且内容物含量稳定[86]。尽管EVs在临床诊断上取得了一些令人鼓舞的成果,但仍然存在诸多挑战,比如需要特殊的仪器,如MiSeq系统和气相色谱以及生物信息学工具来分析获得的数据[87]。为了应对这些挑战,需要更多的研究来减少对技术平台的要求,并使数据分析更容易。预计在不久的将来,随着EVs基础研究和纯化手段的不断进步,EVs有望在临床上成为疾病的特异性诊断工具,而且这一领域的研究工作将有助于理解复杂的微生物-宿主通信网络,这对保护人类健康至关重要。
| [1] |
KALLURI R, LeBLEU VS. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. DOI:10.1126/science.aau6977 |
| [2] |
MCMILLAN HM, KUEHN MJ. The extracellular vesicle generation paradox: a bacterial point of view[J]. The EMBO Journal, 2021, 40(21): e108174. DOI:10.15252/embj.2021108174 |
| [3] |
van NIEL G, D'ANGELO G, RAPOSO G. Shedding light on the cell biology of extracellular vesicles[J]. Nature Reviews Molecular Cell Biology, 2018, 19(4): 213-228. DOI:10.1038/nrm.2017.125 |
| [4] |
PAN BT, JOHNSTONE RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor[J]. Cell, 1983, 33(3): 967-978. DOI:10.1016/0092-8674(83)90040-5 |
| [5] |
ZHANG L, YU DH. Exosomes in cancer development, metastasis, and immunity[J]. Biochimica et Biophysica Acta Reviews on Cancer, 2019, 1871(2): 455-468. DOI:10.1016/j.bbcan.2019.04.004 |
| [6] |
HOEKSTRA D, van der LAAN JW, de LEIJ L, WITHOLT B. Release of outer membrane fragments from normally growing Escherichia coli[J]. Biochimica et Biophysica Acta, 1976, 455(3): 889-899. DOI:10.1016/0005-2736(76)90058-4 |
| [7] |
SARTORIO MG, PARDUE EJ, FELDMAN MF, HAURAT MF. Bacterial outer membrane vesicles: from discovery to applications[J]. Annual Review of Microbiology, 2021, 75: 609-630. DOI:10.1146/annurev-micro-052821-031444 |
| [8] |
TOYOFUKU M, NOMURA N, EBERL L. Types and origins of bacterial membrane vesicles[J]. Nature Reviews Microbiology, 2019, 17(1): 13-24. DOI:10.1038/s41579-018-0112-2 |
| [9] |
MENG LN, CAO XL, ZHANG Y, ZHOU WQ, SHEN H. Research progress of extracellular vesicles of fungi[J]. Chinese Journal of Mycology, 2022, 17(4): 349-352. (in Chinese) 孟玲宁, 曹小利, 张燕, 周万青, 沈瀚. 真菌细胞外囊泡的研究进展[J]. 中国真菌学杂志, 2022, 17(4): 349-352. |
| [10] |
GARCIA-CERON D, BLEACKLEY MR, ANDERSON MA. Fungal extracellular vesicles in pathophysiology[M]//Subcellular Biochemistry. Cham: Springer International Publishing, 2021: 151-177.
|
| [11] |
PROCTOR LM, CREASY HH, FETTWEIS JM, LLOYD-PRICE J, MAHURKAR A, ZHOU WY, BUCK GA, SNYDE MP, STRAUSS JF, WEINSTOCK GM, WHITE O, HUTTENHOWER C. The integrative human microbiome project[J]. Nature, 2019, 569(7758): 641-648. DOI:10.1038/s41586-019-1238-8 |
| [12] |
de VOS WM, TILG H, van HUL M, CANI PD. Gut microbiome and health: mechanistic insights[J]. Gut, 2022, 71(5): 1020-1032. DOI:10.1136/gutjnl-2021-326789 |
| [13] |
URSELL LK, HAISER HJ, van TREUREN W, GARG N, REDDIVARI L, VANAMALA J, DORRESTEIN PC, TURNBAUGH PJ, KNIGHT R. The intestinal metabolome: an intersection between microbiota and host[J]. Gastroenterology, 2014, 146(6): 1470-1476. DOI:10.1053/j.gastro.2014.03.001 |
| [14] |
MA XY, SHIN YJ, YOO JW, PARK HS, KIM DH. Extracellular vesicles derived from Porphyromonas gingivalis induce trigeminal nerve-mediated cognitive impairment[J]. Journal of Advanced Research, 2023. DIO: 10.1016/j. jare. 2023.02. 006.
|
| [15] |
ZHANG ZW, LIU XH, YANG XQ, JIANG Y, LI A, CONG JY, LI YW, XIE QJ, XU C, LIU DB. Identification of faecal extracellular vesicles as novel biomarkers for the non-invasive diagnosis and prognosis of colorectal cancer[J]. Journal of Extracellular Vesicles, 2023, 12(1): 12300. DOI:10.1002/jev2.12300 |
| [16] |
QIAN MY, LIU J, ZHAO DY, PAN CY, JIA WX, GAO YS, ZHANG YF, YANG S, ZHANG N, ZHANG YN, ZHANG Q, WU DL, SCHNABL B, SHEN X, WANG LR. 715 aryl hydrocarbon receptor deficiency in intestinal epithelial cells aggravates alcohol-related liver disease[J]. Gastroenterology, 2021, 160(6): S-794. |
| [17] |
LI Y, ZHAO DY, QIAN MY, LIU J, PAN CY, ZHANG XX, DUAN XB, ZHANG YF, JIA WX, WANG LR. Amlodipine, an anti-hypertensive drug, alleviates non-alcoholic fatty liver disease by modulating gut microbiota[J]. British Journal of Pharmacology, 2022, 179(9): 2054-2077. DOI:10.1111/bph.15768 |
| [18] |
WANG L, FOUTS DE, STÄRKEL P, HARTMANN P, CHEN P, LLORENTE C, DEPEW J, MONCERA K, HO SB, BRENNER DA, HOOPER LV, SCHNABL B. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation[J]. Cell Host and Microbe, 2016, 19(2): 227-239. DOI:10.1016/j.chom.2016.01.003 |
| [19] |
NAKAO Y, AMROLLAHI P, PARTHASARATHY G, MAUER AS, SEHRAWAT TS, VANDERBOOM P, NAIR KS, NAKAO K, ALLEN AM, HU TY, MALHI H. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery[J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2021, 36: 102430. DOI:10.1016/j.nano.2021.102430 |
| [20] |
WU DQ, ZHU HQ, WANG H. Extracellular vesicles in non-alcoholic fatty liver disease and alcoholic liver disease[J]. Frontiers in Physiology, 2021, 12: 707429. DOI:10.3389/fphys.2021.707429 |
| [21] |
TANG SL, XIE ZY. The role of exosomes in liver fibrosis[J]. Chemistry of Life, 2020, 40(2): 230-235. (in Chinese) 汤胜兰, 谢正元. 外泌体在肝纤维化中的作用[J]. 生命的化学, 2020, 40(2): 230-235. DOI:10.13488/j.smhx.20190137 |
| [22] |
FANG CY, WEI YW. Extracellular vesicles in liver pathobiology[J]. Medical Recapitulate, 2017, 23(16): 3142-3145. (in Chinese) 方程远, 魏云巍. 细胞外囊泡在肝脏疾病中的研究现状[J]. 医学综述, 2017, 23(16): 3142-3145. |
| [23] |
THAKUR A, KE XS, CHEN YW, MOTALLEBNEJAD P, ZHANG K, LIAN QZ, CHEN HJ. The mini player with diverse functions: extracellular vesicles in cell biology, disease, and therapeutics[J]. Protein and Cell, 2022, 13(9): 631-654. DOI:10.1007/s13238-021-00863-6 |
| [24] |
JEPPESEN DK, FENIX AM, FRANKLIN JL, HIGGINBOTHAM JN, ZHANG Q, ZIMMERMAN LJ, LIEBLER DC, PING J, LIU Q, EVANS R, FISSELL WH, PATTON JG, ROME LH, BURNETTE DT, COFFEY RJ. Reassessment of exosome composition[J]. Cell, 2019, 177(2): 428-445.e18. DOI:10.1016/j.cell.2019.02.029 |
| [25] |
DIXSON AC, DAWSON TR, di VIZIO D, WEAVER AM. Context-specific regulation of extracellular vesicle biogenesis and cargo selection[J]. Nature Reviews Molecular Cell Biology, 2023, 24(7): 454-476. DOI:10.1038/s41580-023-00576-0 |
| [26] |
SCHWECHHEIMER C, SULLIVAN CJ, KUEHN MJ. Envelope control of outer membrane vesicle production in gram-negative bacteria[J]. Biochemistry, 2013, 52(18): 3031-3040. DOI:10.1021/bi400164t |
| [27] |
SANTOS JC, DICK MS, LAGRANGE B, DEGRANDI D, PFEFFER K, YAMAMOTO M, MEUNIER E, PELCZAR P, HENRY T, BROZ P. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation[J]. The EMBO Journal, 2018, 37(6): e98089. DOI:10.15252/embj.201798089 |
| [28] |
DEATHERAGE BL, LARA JC, BERGSBAKEN T, RASSOULIAN BARRETT SL, LARA S, COOKSON BT. Biogenesis of bacterial membrane vesicles[J]. Molecular Microbiology, 2009, 72(6): 1395-1407. DOI:10.1111/j.1365-2958.2009.06731.x |
| [29] |
WENSINK J, WITHOLT B. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein[J]. European Journal of Biochemistry, 1981, 116(2): 331-335. DOI:10.1111/j.1432-1033.1981.tb05338.x |
| [30] |
SCHWECHHEIMER C, KULP A, KUEHN MJ. Modulation of bacterial outer membrane vesicle production by envelope structure and content[J]. BMC Microbiology, 2014, 14: 324. DOI:10.1186/s12866-014-0324-1 |
| [31] |
JIANG YL, KONG QK, ROLAND KL, Curtiss R. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity[J]. International Journal of Medical Microbiology, 2014, 304(3/4): 431-443. |
| [32] |
MASHBURN LM, WHITELEY M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote[J]. Nature, 2005, 437(7057): 422-425. DOI:10.1038/nature03925 |
| [33] |
MASHBURN-WARREN L, HOWE J, BRANDENBURG K, WHITELEY M. Structural requirements of the Pseudomonas quinolone signal for membrane vesicle stimulation[J]. Journal of Bacteriology, 2009, 191(10): 3411-3414. DOI:10.1128/JB.00052-09 |
| [34] |
MCBROOM AJ, JOHNSON AP, VEMULAPALLI S, KUEHN MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability[J]. Journal of Bacteriology, 2006, 188(15): 5385-5392. DOI:10.1128/JB.00498-06 |
| [35] |
MCBROOM AJ, KUEHN MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response[J]. Molecular Microbiology, 2007, 63(2): 545-558. DOI:10.1111/j.1365-2958.2006.05522.x |
| [36] |
SCHERTZER JW, WHITELEY M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis[J]. mBio, 2012, 3(2): e00297-11. |
| [37] |
SCHWECHHEIMER C, KUEHN MJ. Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions[J]. Nature Reviews Microbiology, 2015, 13(10): 605-619. DOI:10.1038/nrmicro3525 |
| [38] |
LEE EY, CHOI DY, KIM DK, KIM JW, PARK JO, KIM S, KIM SH, DESIDERIO DM, KIM YK, KIM KP, GHO YS. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles[J]. Proteomics, 2009, 9(24): 5425-5436. DOI:10.1002/pmic.200900338 |
| [39] |
van BALKOM BW, EISELE AS, PEGTEL DM, BERVOETS S, VERHAAR MC. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting[J]. Journal of Extracellular Vesicles, 2015, 4: 26760. DOI:10.3402/jev.v4.26760 |
| [40] |
KEERTHIKUMAR S, CHISANGA D, ARIYARATNE D, AL SAFFAR H, ANAND S, ZHAO KN, SAMUEL M, PATHAN M, JOIS M, CHILAMKURTI N, GANGODA L, MATHIVANAN S. ExoCarta: a web-based compendium of exosomal cargo[J]. Journal of Molecular Biology, 2016, 428(4): 688-692. DOI:10.1016/j.jmb.2015.09.019 |
| [41] |
PATHAN M, FONSEKA P, CHITTI SV, KANG T, SANWLANI R, van DEUN J, HENDRIX A, MATHIVANAN S. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles[J]. Nucleic Acids Research, 2019, 47(D1): D516-D519. DOI:10.1093/nar/gky1029 |
| [42] |
ZHANG RR, HU YY, YUAN JY, WU DZ. Effects of Puerariae radix extract on the increasing intestinal permeability in rat with alcohol-induced liver injury[J]. Journal of Ethnopharmacology, 2009, 126(2): 207-214. DOI:10.1016/j.jep.2009.08.044 |
| [43] |
KUEHN MJ, KESTY NC. Bacterial outer membrane vesicles and the host-pathogen interaction[J]. Genes & Development, 2005, 19(22): 2645-2655. |
| [44] |
BEVERIDGE TJ. Structures of gram-negative cell walls and their derived membrane vesicles[J]. Journal of Bacteriology, 1999, 181(16): 4725-4733. DOI:10.1128/JB.181.16.4725-4733.1999 |
| [45] |
PETTIT RK, JUDD RC. Characterization of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae[J]. Molecular Microbiology, 1992, 6(6): 723-728. DOI:10.1111/j.1365-2958.1992.tb01521.x |
| [46] |
GRENIER D, MAYRAND D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis[J]. Infection and Immunity, 1987, 55(1): 111-117. DOI:10.1128/iai.55.1.111-117.1987 |
| [47] |
KATO S, KOWASHI Y, DEMUTH DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin[J]. Microbial Pathogenesis, 2002, 32(1): 1-13. DOI:10.1006/mpat.2001.0474 |
| [48] |
HORSTMAN AL, KUEHN MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles[J]. The Journal of Biological Chemistry, 2000, 275(17): 12489-12496. DOI:10.1074/jbc.275.17.12489 |
| [49] |
ALTINDIS E, FU Y, MEKALANOS JJ. Proteomic analysis of Vibrio cholerae outer membrane vesicles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(15): E1548-E1556. |
| [50] |
CHOI DS, KIM DK, CHOI SJ, LEE J, CHOI JP, RHO S, PARK SH, KIM YK, HWANG D, GHO YS. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa[J]. Proteomics, 2011, 11(16): 3424-3429. DOI:10.1002/pmic.201000212 |
| [51] |
RIVERA J, CORDERO RJB, NAKOUZI AS, FRASES S, NICOLA A, CASADEVALL A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(44): 19002-19007. |
| [52] |
OLAYA-ABRIL A, PRADOS-ROSALES R, MCCONNELL MJ, MARTÍN-PEÑA R, GONZÁLEZ- REYES JA, JIMÉNEZ-MUNGUÍA I, GÓMEZ- GASCÓN L, FERNÁNDEZ J, LUQUE-GARCÍA JL, GARCÍA-LIDÓN C, ESTÉVEZ H, PACHÓN J, OBANDO I, CASADEVALL A, PIROFSKI LA, RODRÍGUEZ-ORTEGA MJ. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae[J]. Journal of Proteomics, 2014, 106: 46-60. DOI:10.1016/j.jprot.2014.04.023 |
| [53] |
LARIOS J, MERCIER V, ROUX A, GRUENBERG J. ALIX- and ESCRT-Ⅲ-dependent sorting of tetraspanins to exosomes[J]. Journal of Cell Biology, 2020, 219(3): e201904113. |
| [54] |
CHEN YD, FANG YT, CHENG YL, LIN CF, HSU LJ, WANG SY, ANDERSON R, CHANG CP, LIN YS. Exophagy of annexin A2 via RAB11, RAB8A and RAB27A in IFN-γ-stimulated lung epithelial cells[J]. Scientific Reports, 2017, 7: 5676. DOI:10.1038/s41598-017-06076-4 |
| [55] |
JIANG LL, SHEN YY, GUO DF, YANG DY, LIU JJ, FEI XF, YANG YS, ZHANG BY, LIN ZD, YANG F, WANG XJ, WANG KY, WANG JL, CAI ZJ. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance[J]. Nature Communications, 2016, 7: 13045. DOI:10.1038/ncomms13045 |
| [56] |
BÜNING J, von SMOLINSKI D, TAFAZZOLI K, ZIMMER KP, STROBEL S, APOSTOLAKI M, KOLLIAS G, HEATH JK, LUDWIG D, GEBERT A. Multivesicular bodies in intestinal epithelial cells: responsible for MHC class Ⅱ-restricted antigen processing and origin of exosomes[J]. Immunology, 2008, 125(4): 510-521. DOI:10.1111/j.1365-2567.2008.02864.x |
| [57] |
BENINSON LA, FLESHNER M. Exosomes: an emerging factor in stress-induced immunomodulation[J]. Seminars in Immunology, 2014, 26(5): 394-401. DOI:10.1016/j.smim.2013.12.001 |
| [58] |
el ANDALOUSSI S, MÄGER I, BREAKEFIELD XO, WOOD MJA. Extracellular vesicles: biology and emerging therapeutic opportunities[J]. Nature Reviews Drug Discovery, 2013, 12(5): 347-357. DOI:10.1038/nrd3978 |
| [59] |
BRIAUD P, CARROLL RK. Extracellular vesicle biogenesis and functions in gram-positive bacteria[J]. Infection and Immunity, 2020, 88(12): e00433-20. |
| [60] |
TOYOFUKU M, SCHILD S, KAPARAKIS-LIASKOS M, EBERL L. Composition and functions of bacterial membrane vesicles[J]. Nature Reviews Microbiology, 2023, 21(7): 415-430. DOI:10.1038/s41579-023-00875-5 |
| [61] |
CHAMPAGNE-JORGENSEN K, MIAN MF, MCVEY NEUFELD KA, STANISZ AM, BIENENSTOCK J. Membrane vesicles of Lacticaseibacillus rhamnosus JB-1 contain immunomodulatory lipoteichoic acid and are endocytosed by intestinal epithelial cells[J]. Scientific Reports, 2021, 11: 13756. DOI:10.1038/s41598-021-93311-8 |
| [62] |
BIAGINI M, GARIBALDI M, APREA S, PEZZICOLI A, DORO F, BECHERELLI M, TADDEI AR, TANI C, TAVARINI S, MORA M, TETI G, D'ORO U, NUTI S, SORIANI M, MARGARIT I, RAPPUOLI R, GRANDI G, NORAIS N. The human pathogen Streptococcus pyogenes releases lipoproteins as lipoprotein-rich membrane vesicles[J]. Molecular & Cellular Proteomics: MCP, 2015, 14(8): 2138-2149. |
| [63] |
LEE AR, BIN PARK S, KIM SW, JUNG JW, CHUN JH, KIM J, KIM YR, LAZARTE JMS, BIN JANG H, THOMPSON KD, JUNG M, HA MW, JUNG TS. Membrane vesicles from antibiotic-resistant Staphylococcus aureus transfer antibiotic-resistance to antibiotic-susceptible Escherichia coli[J]. Journal of Applied Microbiology, 2022, 132(4): 2746-2759. DOI:10.1111/jam.15449 |
| [64] |
JERMY A. eDNA limits biofilm attachment[J]. Nature Reviews Microbiology, 2010, 8(9): 612-613. |
| [65] |
OKSHEVSKY M, REGINA VR, MEYER RL. Extracellular DNA as a target for biofilm control[J]. Current Opinion in Biotechnology, 2015, 33: 73-80. DOI:10.1016/j.copbio.2014.12.002 |
| [66] |
SAHU PK, IYER PS, OAK AM, PARDESI KR, CHOPADE BA. Characterization of eDNA from the clinical StrainAcinetobacter baumanniiAIIMS 7 and its role in biofilm formation[J]. The Scientific World Journal, 2012, 2012: 1-10. |
| [67] |
JONES EJ, BOOTH C, FONSECA S, PARKER A, CROSS K, MIQUEL-CLOPÉS A, HAUTEFORT I, MAYER U, WILEMAN T, STENTZ R, CARDING SR. The uptake, trafficking, and biodistribution of Bacteroides thetaiotaomicron generated outer membrane vesicles[J]. Frontiers in Microbiology, 2020, 11: 57. DOI:10.3389/fmicb.2020.00057 |
| [68] |
KIM JH, YOON YJ, LEE J, CHOI EJ, YI N, PARK KS, PARK J, LÖTVALL J, KIM YK, GHO YS. Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo[J]. PLoS One, 2013, 8(3): e59276. DOI:10.1371/journal.pone.0059276 |
| [69] |
SHAH B, SULLIVAN CJ, LONERGAN NE, STANLEY S, SOULT MC, BRITT LD. Circulating bacterial membrane vesicles cause Sepsis in rats[J]. Shock, 2012, 37(6): 621-628. DOI:10.1097/SHK.0b013e318250de5d |
| [70] |
ZHOU BL, YUAN YT, ZHANG SS, GUO C, LI XL, LI GY, XIONG W, ZENG ZY. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract[J]. Frontiers in Immunology, 2020, 11: 575. DOI:10.3389/fimmu.2020.00575 |
| [71] |
DÍAZ-GARRIDO N, BADIA J, BALDOMÀ L. Microbiota-derived extracellular vesicles in interKingdom communication in the gut[J]. Journal of Extracellular Vesicles, 2021, 10(13): e12161. DOI:10.1002/jev2.12161 |
| [72] |
IRVING T, MIMURO H, KUFER T, LO C, WHEELER R, TURNER LJ, THOMAS BJ, MALOSSE C, GANTIER MP, CASILLAS LN, VOTTA BJ, BERTIN J, BONECA IG, SASAKAWA C, PHILPOTT DJ, FERRERO RL, KAPARAKIS-LIASKOS M. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling[J]. Cell Host and Microbe, 2014, 15(5): 623-635. DOI:10.1016/j.chom.2014.04.001 |
| [73] |
BITTEL M, REICHERT P, SARFATI I, DRESSEL A, LEIKAM S, UDERHARDT S, STOLZER I, PHU TA, NG M, VU NK, TENZER S, DISTLER U, WIRTZ S, ROTHHAMMER V, NEURATH MF, RAFFAI RL, GÜNTHER C, MOMMA S. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo[J]. Journal of Extracellular Vesicles, 2021, 10(12): e12159. DOI:10.1002/jev2.12159 |
| [74] |
KANG M, JORDAN V, BLENKIRON C, CHAMLEY LW. Biodistribution of extracellular vesicles following administration into animals: a systematic review[J]. Journal of Extracellular Vesicles, 2021, 10(8): e12085. DOI:10.1002/jev2.12085 |
| [75] |
FIZANNE L, VILLARD A, BENABBOU N, RECOQUILLON S, SOLETI R, DELAGE E, WERTHEIMER M, VIDAL-GÓMEZ X, OULLIER T, CHAFFRON S, MARTÍNEZ MC, NEUNLIST M, BOURSIER J, ANDRIANTSITOHAINA R. Faeces-derived extracellular vesicles participate in the onset of barrier dysfunction leading to liver diseases[J]. Journal of Extracellular Vesicles, 2023, 12(2): 12303. DOI:10.1002/jev2.12303 |
| [76] |
KUMAR A, SUNDARAM K, MU JY, DRYDEN GW, SRIWASTVA MK, LEI C, ZHANG LF, QIU XL, XU FY, YAN J, ZHANG X, PARK JW, MERCHANT ML, BOHLER HCL, WANG BM, ZHANG SQ, QIN C, XU ZY, HAN XL, MCCLAIN CJ, et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance[J]. Nature Communications, 2021, 12: 213. DOI:10.1038/s41467-020-20500-w |
| [77] |
LUO Z, JI Y, GAO H, REIS FCGD, BANDYOPADHYAY G, JIN Z, LY C, CHANG Y, ZHANG D, KUMAR D, YING W. CRIg+ macrophages prevent gut microbial DNA-containing extracellular vesicle-induced tissue inflammation and insulin resistance[J]. Gastroenterology, 2021, 160(3): 863-874. DOI:10.1053/j.gastro.2020.10.042 |
| [78] |
LUO ZL, JI YD, ZHANG DH, GAO H, JIN ZM, YANG MX, YING W. Microbial DNA enrichment promotes liver steatosis and fibrosis in the course of non-alcoholic steatohepatitis[J]. Acta Physiologica, 2022, 235(3): e13827. DOI:10.1111/apha.13827 |
| [79] |
ZAHMATKESH ME, JAHANBAKHSH M, HOSEINI N, SHEGEFTI S, PEYMANI A, DABIN H, SAMIMI R, BOLORI S. Effects of exosomes derived from Helicobacter pylori outer membrane vesicle-infected hepatocytes on hepatic stellate cell activation and liver fibrosis induction[J]. Frontiers in Cellular and Infection Microbiology, 2022, 12: 857570. DOI:10.3389/fcimb.2022.857570 |
| [80] |
KESHAVARZ AZIZI RAFTAR S, ASHRAFIAN F, YADEGAR A, LARI A, MORADI HR, SHAHRIARY A, AZIMIRAD M, ALAVIFARD H, MOHSENIFAR Z, DAVARI M, VAZIRI F, MOSHIRI A, DAVAR SIADAT S, ZALI MR. The protective effects of live and pasteurized Akkermansia muciniphila and its extracellular vesicles against HFD/CCl4-induced liver injury[J]. Microbiology Spectrum, 2021, 9(2): e00484-21. |
| [81] |
TULKENS J, VERGAUWEN G, van DEUN J, GEEURICKX E, DHONDT B, LIPPENS L, de SCHEERDER MA, MIINALAINEN I, RAPPU P, de GEEST BG, VANDECASTEELE K, LAUKENS D, VANDEKERCKHOVE L, DENYS H, VANDESOMPELE J, de WEVER O, HENDRIX A. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction[J]. Gut, 2020, 69(1): 191-193. DOI:10.1136/gutjnl-2018-317726 |
| [82] |
KIM SI, KANG N, LEEM S, YANG J, JO H, LEE M, KIM HS, DHANASEKARAN DN, KIM YK, PARK T, SONG yong sang. Metagenomic analysis of serum microbe-derived extracellular vesicles and diagnostic models to differentiate ovarian cancer and benign ovarian tumor[J]. Cancers, 2020, 12(5): 1309. DOI:10.3390/cancers12051309 |
| [83] |
WEI SC, WEI W, PENG WJ, LIU Z, CAI ZY, ZHAO B. Metabolic alterations in the outer membrane vesicles of patients with alzheimer's disease: an LC-MS/MS-based metabolomics analysis[J]. Current Alzheimer Research, 2020, 16(13): 1183-1195. DOI:10.2174/1567205016666191121141352 |
| [84] |
LEE YS, KIM JH, LIM DH. Urine microbe-derived extracellular vesicles in children with asthma[J]. Allergy, Asthma & Immunology Research, 2021, 13(1): 75. |
| [85] |
SAMRA MS, LIM DH, HAN man yong, JEE HM, KIM YK, KIM JH. Bacterial microbiota-derived extracellular vesicles in children with allergic airway diseases: compositional and functional features[J]. Allergy, Asthma & Immunology Research, 2021, 13(1): 56. |
| [86] |
QIN B, ZHANG Q, HU XM, MI TY, YU HY, LIU SS, ZHANG B, TANG M, HUANG JF, XIONG K. How does temperature play a role in the storage of extracellular vesicles?[J]. Journal of Cellular Physiology, 2020, 235(11): 7663-7680. DOI:10.1002/jcp.29700 |
| [87] |
THIETART S, RAUTOU PE. Extracellular vesicles as biomarkers in liver diseases: a clinician's point of view[J]. Journal of Hepatology, 2020, 73(6): 1507-1525. DOI:10.1016/j.jhep.2020.07.014 |
 2023, Vol. 50
2023, Vol. 50