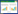扩展功能
文章信息
- 李双青, 肖燕, 王鑫威, 姜海波
- LI Shuangqing, XIAO Yan, WANG Xinwei, JIANG Haibo
- 固氮蓝细菌束毛藻生物固氮策略研究进展
- Progress in the nitrogen fixation strategy of Trichodesmium
- 微生物学通报, 2023, 50(8): 3606-3619
- Microbiology China, 2023, 50(8): 3606-3619
- DOI: 10.13344/j.microbiol.china.230090
-
文章历史
- 收稿日期: 2023-02-10
- 接受日期: 2023-04-14
- 网络首发日期: 2023-05-23
2. 华中师范大学生命科学学院, 湖北 武汉 430079;
3. 南方海洋科学与工程广东省实验室(珠海), 广东 珠海 519080
2. School of Life Sciences, Central China Normal University, Wuhan 430079, Hubei, China;
3. Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai 519080, Guangdong, China
束毛藻(Tricodesmium)是一类不产生异形胞的海洋固氮蓝细菌(蓝藻),属于蓝藻门(Cyanophyta)中的颤藻目(Oscillatoriales),广泛分布于热带和亚热带海洋的寡营养海域表层中[1-3]。束毛藻是海洋中已知数量最多的一类固氮蓝细菌,它们通过固定N2从而减轻寡营养海域的氮限制。研究表明,束毛藻每年固定大约100 Tg的氮,占全球生物固氮的42%,同时它们能将自身固定的大约30%−50%的氮以铵(NH4+)和溶解有机氮(dissolved organic nitrogen, DON)的形式释放至周围环境中[4-5]。束毛藻释放的氮被其他非固氮浮游植物利用,间接驱动海洋初级生产力,在全球的碳、氮循环中起着关键作用[1, 6]。
氮(N)、磷(P)和铁(Fe)元素是海洋初级生产力的主要限制性营养元素[7-9]。氮作为生物所必需的大量元素,在自然界中常见的存在形式有N2、氨(NH4+)、硝酸盐(NO3−)、亚硝酸盐(NO2−)以及有机氮(例如氨基酸和尿素)等[10]。海洋中“新氮”主要来自漩涡扩散(eddy diffusion)、径流输入、大气沉降以及生物固氮作用[11-13]。海水中溶解的N2是海洋中最丰富的氮形式,却很难直接被生物所用,需要固氮生物将N2转化为化合态氮才能进入生物地球化学循环[1, 14]。维持海洋生态系统的氮循环需要大量新生氮的输入,其中固氮蓝细菌所固定的N2占了很大一部分[8, 15-17]。研究表明,固氮生物中的关键酶固氮酶(nitrogenase)在有氧条件下会不可逆地失活[18]。因此,固氮生物必须具有一套保护固氮酶免受氧损伤的机制。一般固氮蓝细菌通过形成异形胞,在空间上将产氧光合作用与固氮作用区分开,避免固氮酶受到氧气的攻击。然而束毛藻在长期进化适应过程中形成了一套独特的机制,使其在不产生异形胞的情况下进行光合作用和固氮作用,进而在海洋氮循环中发挥重要作用,成为海洋最主要的固氮微生物。束毛藻的生物固氮策略在海洋固氮生物中极具代表性和独特性,深入揭示该固氮生物的运行模式和调控机制对全面认识海洋氮循环具有重要意义。
近年来,人们对束毛藻的生物固氮机制已经有了较为深入的研究,本文全面综述了束毛藻固氮和光合的“时空分离”机制,总结了束毛藻生物固氮的环境调控和响应机制,以便进一步深入了解束毛藻的独特固氮特性以及海洋氮循环的内在运行规律。
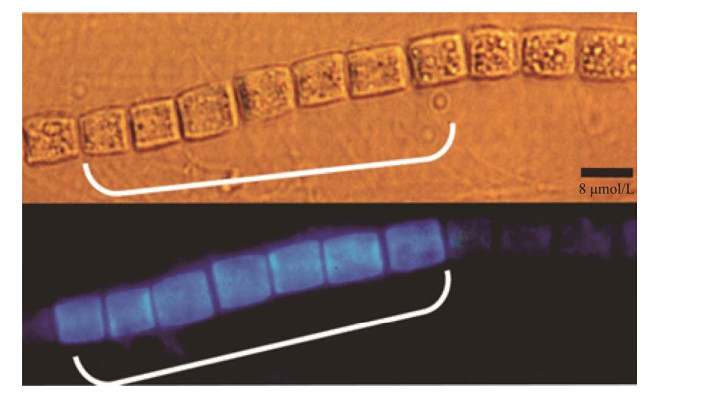
1 束毛藻在海洋氮循环中的作用海洋中已知的常见固氮微生物主要有3个大类:不具有异形胞的丝状束毛藻,单细胞固氮蓝细菌鳄球藻(Crocosphaera),以及与单细胞真核藻类(Richelia、Calothrix等)形成共生体的蓝细菌[2, 19-20]。此外还有近年来新发现的单细胞共生UCYN-A和非蓝细菌固氮细菌等[21-23]。束毛藻是海洋固氮蓝细菌中最主要的类群,在海洋中通常以菌落形式存在,并且具有高光强、弱垂直混合和低溶解无机氮(dissolved inorganic nitrogen, DIN)浓度的海域分布特征[24-25]。自然海域束毛藻群落固定的氮大部分以溶解有机氮(DON)的形式释放出来[24, 26]。首先,束毛藻将大气中的N2固定为有机氮或无机氮形式,然后这些形式的氮会在束毛藻细胞之间进行转换与利用,供营养细胞(非固氮细胞)所用[27]。然后通过自身细胞程序性死亡或病毒诱导的裂解将氮释放到周围环境中,供周围微生物所用或经捕食者(滤食性动物、浮游动物、杂食性动物和鱼类)进入食物链被利用[4, 24, 28]。尤其是在束毛藻水华时期,束毛藻可为海洋提供丰富的可利用性氮源[4]。如图 1所示,以束毛藻为主的生物固氮作用(将N2→结合态氮)代表了海洋主要氮输入过程,对于维持海洋的氮平衡具有重要作用[14, 21, 29-30]。
|
| 图 1 固氮蓝细菌在开阔海洋氮循环中的作用示意图 Figure 1 Schematic diagram of the role of nitrogen-fixing cyanobacteria in the nitrogen cycle of the open ocean. |
|
|
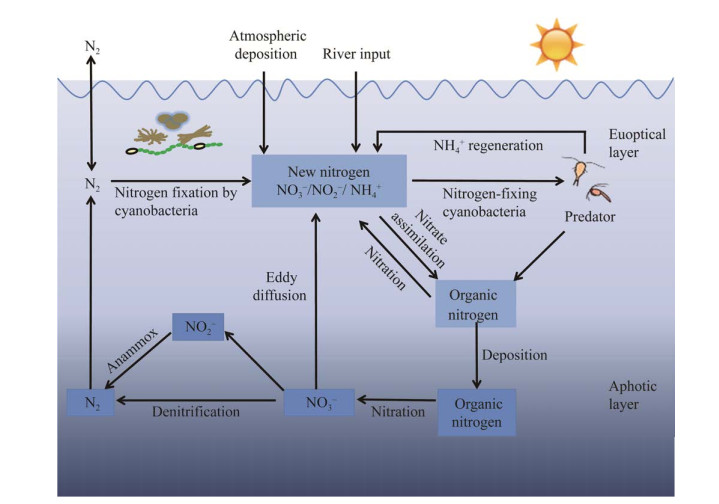
生物固氮的基本反应为:N2+8H++16ATP+ 8e–→2NH3+H2+16ADP+16Pi,该反应由固氮酶复合物催化,固氮酶具有钼(Mo)依赖型(NIF)、钒(V)依赖型(VNF)、铁(Fe)依赖型或异质金属依赖型(ANF)[31]。蓝细菌所具有的固氮酶复合物是最常见的NIF型,由固氮酶(钼铁蛋白)和固氮还原酶(铁蛋白)组成,由nifH、nifD和nifK基因编码[30, 32-34]。不同蓝细菌中的固氮酶基因具有高度的保守性,且无论是在转录和蛋白水平都对氧气非常敏感[35]。固氮作用是一个极为耗能的过程,对固氮蓝细菌来说,绝大部分的能量来源于自身碳固定(光合作用),如Crocosphaera watsonii WH 8501等异形胞固氮蓝藻会进行光合作用储存大量的糖原作为其在固氮时的能量储备[6, 36]。如图 2所示:光合作用和呼吸作用为固氮过程提供了能量(ATP)和还原剂(NADPH)[26, 37];呼吸作用消耗光合作用产生的氧气,对保护固氮酶免受O2破坏具有正面作用。
|
| 图 2 蓝细菌固氮、光合及呼吸之间的相互作用示意图 Figure 2 Schematic diagram of the interaction between nitrogen fixation, photosynthesis, and respiration in Tricodesmium. The green part of the diagram shows the photosynthetic apparatus, the red-brown part shows the respiratory apparatus and carbon fixation, and the apparatus common to respiration and photosynthesis is shown in red-brown and green together. The blue box shows nitrogen fixation, which does not occur in the same compartment as photosynthesis. The orange box shows the Mehler reaction. The dashed arrows in the electron transport chain involving photosynthesis and respiration indicate electron transport and the solid arrows indicate proton (H+) transfer. APX: Ascorbate peroxidase; Cytb6f: Cytochrome b6f complex; Cytc oxidase: Cytochrome C oxidase; Fd: Ferredoxin; NDH-1: NAD(P)H dehydrogenase; PC: Plastocyanin; PQ: Plastoquinone; PSI: Photosystem I; PSII: Photosystem II; Succin: Succinate dehydrogenase; SOD: Superoxide dismutases. 该图中绿色部分表示光合装置,红棕色部分表示呼吸装置和碳固定,呼吸和光合共有的装置则用红棕色和绿色共同表示. 该图蓝色框内显示的是固氮作用,固氮与光合作用不是发生在同一区室内. 橙色框内表示的是梅勒(Mehler)反应. 涉及光合和呼吸电子传递链中的虚线箭头表示的是电子传递,实线箭头表现的是质子(H+)传递. APX:抗坏血酸过氧化物酶;Cytb6f:细胞色素b6f复合物;Cytc oxidase:细胞色素C氧化酶;Fd:铁氧还蛋白;NDH-1:NAD(P)H脱氢酶;PC:质体蓝素;PQ:质体醌;PSI:光系统I;PSII:光系统II;Succinase:琥珀酸脱氢酶;SOD:超氧化物歧化酶 |
|
|
大量研究表明,固氮细胞周围空间的氧气浓度越高,其固氮作用受到的抑制程度就越大,即氧气浓度与固氮速率成反比关系[18, 38]。因此,固氮蓝细菌在长期的进化过程中演化出一套独特的固氮策略,保证其在有氧的海洋环境中进行正常固氮。通常情况下,固氮细菌通过将光合作用和固氮作用分离来实现在有氧环境中进行固氮这一目的。例如,(1) 在空间上分离,产异形胞固氮蓝细菌通过分化成高度特化的异形胞实现与光合作用的分隔(如鱼腥藻Anabeana等);(2) 在时间上分离,通过在夜间固定氮,白天进行光合作用(通常见于单细胞固氮蓝细菌Crocosphaera)实现二者的分隔[26, 37, 39] (图 3)。

|
| 图 3 两种模式固氮蓝细菌(丝状固氮蓝藻Anabeana和单细胞固氮蓝细菌Crocosphaera)的不同固氮方式、固氮作用和光合作用的分离策略 Figure 3 Different nitrogen-fixing morphology, separation of nitrogen-fixing effects and photosynthesis of two model organisms (Filamentous Anabaena and unicellular nitrogen-fixing cyanobacteria-Crocosphaera). Blue indicates localization of intracellular nitrogenase, and green indicates vegetative cells that are actively photosynthesizing. 蓝色表示细胞内固氮酶的定位,绿色表示正在积极地进行光合作用的营养细胞 |
|
|
在过去的40多年中,异形胞成为所有固氮蓝细菌N2固定的“共识模型”[40]。然而束毛藻却是一个特例,其自身并无异形胞却能够在光周期内发生N2固定,并且束毛藻的N2固定效率和其他固氮蓝藻相似甚至更高,这引起了研究人员的广泛关注[41]。1976年,Carpenter等[42]通过显微镜发现了束毛藻藻丝中有一段短串固氮细胞(diazocytes),这类细胞占整个束毛藻藻丝的15%−20%。束毛藻的diazocytes与其他蓝藻的异形胞不同之处在于异形胞是分化完全的细胞,不能再进行分裂,而diazocytes的分化是可逆的,可进行正常的生长和分裂繁殖[43];Diazocytes内含有RubisCO酶及所有藻胆体,保留全部PSII,而异形胞中无RubisCO酶,无放氧复合体,仅有部分异形胞会保留藻胆体[43]。2003年,El-Shehawy等[44]发现这类diazocytes不仅含有光系统Ⅰ (PSI)和光系统Ⅱ (PSⅡ),保留了营养细胞的许多光合作用特性,并且还证明束毛藻中的固氮酶位于这类diazocytes中(图 4)。
束毛藻的固氮酶(钼铁蛋白)由nifD和nifK基因编码,与nifH基因编码的固氮还原酶(铁蛋白)共同组成固氮酶复合物发挥功能,将N2催化还原为NH3,反应产物NH3再通过谷氨酰胺合成酶/谷氨酸合成酶(GS/GOGAT)途径进一步同化成谷氨酰胺(glutamine)和谷氨酸(glutamate)[30]。为了减少氧气对这一过程产生的影响,束毛藻利用diazocytes为生物固氮提供了类似不完全“空间分离”的保护策略,但由于diazocytes无厚的细胞壁,其周围营养细胞释放出的O2还是会对固氮酶活性产生影响[42]。2019年Inomura等[41]研究表明,束毛藻藻丝的固氮细胞与营养细胞之间还存在“缓冲细胞”,此类细胞与固氮细胞都保持着很高的呼吸速率,对周围营养细胞的O2扩散至固氮细胞有减缓阻挡作用(图 5)。此外,束毛藻藻丝与藻丝之间存在胞外聚合物,也可阻碍周围环境中的O2扩散至固氮细胞[41]。近期研究表明,大多数束毛藻藻丝中普遍存在一类类胡萝卜素脂质(hopanoid lipids),能够降低细胞外O2进入细胞的通透性[45]。这种独特的不完全“空间分离”策略既保证了束毛藻在有氧的海洋环境中大量生长,同时也可以高效地进行生物固氮[46]。图 5展示了束毛藻的生物固氮在空间上与光合作用的隔离策略。

|
| 图 5 束毛藻在空间上隔离O2的固氮策略 Figure 5 The nitrogen fixation strategy of Trichodesmium spatially segregating O2. |
|
|
2001年,Berman-Frank等[47-48]研究发现,束毛藻的生物固氮除了空间隔离之外还有时间上的分隔。束毛藻的固氮酶活性受光照的调节,所以固氮遵循着昼夜节律,仅在光照阶段发生。这种在光照阶段进行固氮所带来的好处就是可以使颗粒有机碳和颗粒有机氮的生产达到平衡[49]。同时光合作用产生的能量可以直接向固氮酶供能,抵消束毛藻在固氮时所消耗的能量成本,从而减少对糖原的需要,降低细胞密度与下沉速度,使束毛藻成为了一类有浮力、高光适应型的群体,与使用糖原供能的异形胞蓝藻相比在生态环境中占据了更加有利的地位[36, 49]。但是为了能够避免固氮受到光合放氧的影响,束毛藻采取独特的时间间隔策略以错开固氮与光合作用的高峰(图 6)[36]。束毛藻的固氮酶在每天早晨从头合成,在午后和深夜失活降解[40]。固氮速率在光照开始时迅速增加,在中午达到峰值,从下午开始下降,到晚上固氮速率几乎为零,直到次日早晨[2, 24, 41]。相反地,束毛藻光合作用最大值发生于光周期的早期阶段,当O2积累期间达到某个阈值就会引起O2浓度降低,光合作用速率在这一阶段急速下降;在午后光合作用速率又逐渐上升,然后随着光照强度的减弱再次降低,直至日落无光照时降为零[50]。束毛藻固氮峰值出现在光照周期的中间时段,此时较低的光合速率减少了氧气的释放,同时光照前期光合作用释放的大量能量可以保证固氮的正常运行[47-48]。
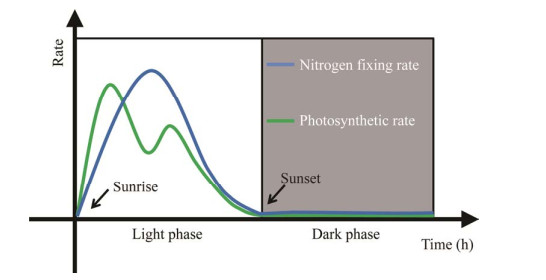
|
| 图 6 束毛藻的生物固氮与光合作用在时间上的分隔示意图(束毛藻群落水平) Figure 6 Separation of nitrogen fixation and photosynthesis of Tricodesmium in time (Tricodesmium community level). This schematic diagram represents the overall trend of measured nitrogen fixation and photosynthetic activity, showing a circadian rhythm[2, 24, 37, 41]. 该示意图表示测定到的固氮和光合活性的总体趋势,显示有昼夜周期节律[2, 24, 37, 41] |
|
|
在N2固定期间,束毛藻群落表现出高呼吸速率,这表明当N2固定和光合作用同时发生时,额外的O2消耗反应会增强[51]。在光合作用中,依赖于光的Mehler反应会消耗PSII所依赖的氧气,这表明依赖于光的氧气消耗机制也可以保护固氮酶[47-48, 52]。由此可知,束毛藻在时间上通过错开固氮与光合高峰并保持高的呼吸速率和Mehler反应以快速消耗多余O2,进而完成高效的生物固氮。
5 束毛藻生物固氮的调节机制除了氧气浓度,束毛藻固氮过程中还会受到其他因素的影响,包括昼夜节律的控制以及光照、温度、CO2浓度、氮源、营养元素的调节[3]。本文将调节束毛藻生物固氮的环境因子总结如图 7所示。
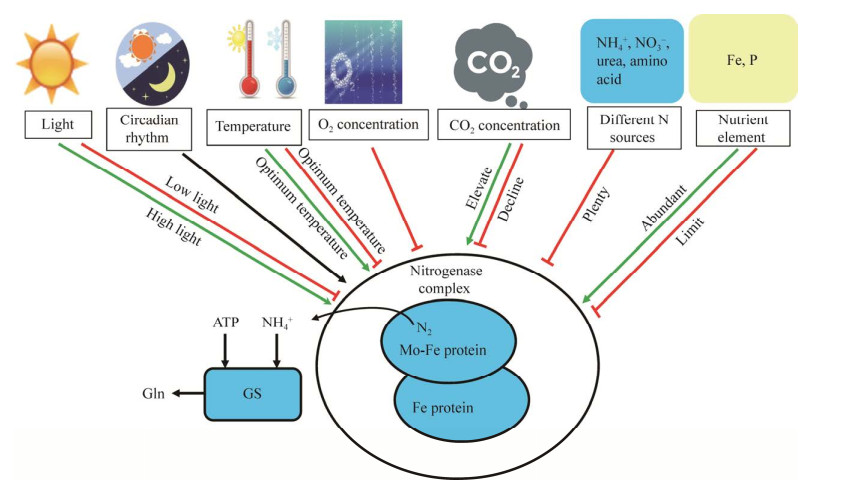
|
| 图 7 束毛藻生物固氮所受的调节因素 Figure 7 The regulatory factors on the nitrogen fixation of Tricodesmium. Green arrow: Promotes nitrogen fixation; Red line: Inhibits nitrogen fixation. GS: Glutamine synthetase. 绿色箭头:促进固氮;红色线:抑制固氮. GS:谷氨酰胺合成酶 |
|
|
研究表明,浮游植物上浮下沉、浮游动物的摄食等行为都显示出昼夜节律,并且昼夜节律在丝状固氮蓝细菌中被证实是一种内源性节律[53]。束毛藻具有控制昼夜节律的基因(kaiABC-生物钟基因),固氮作用基因nifHDK、光合作用基因psbA和psaA的转录都会受到昼夜节律的调控[6, 36, 54]。nifH的翻译后修饰及固氮酶发挥活性都会受到昼夜节律的调控[40]。束毛藻通过昼夜节律的调控可使其在中午光合作用降低时维持最大的固氮酶活性,再加上呼吸作用的稳定增加,维持了适当的氧气浓度,可使固氮过程正常进行[4]。
5.2 光与温度对固氮酶活性的调节束毛藻自然种群在高光强、层化现象明显的热带及亚热带的表层海域中广泛分布,仅在白天进行固氮。Cai等[6]研究表明,高强度光照能增加固氮酶中铁蛋白合成和具有活性的铁蛋白数量,从而促进固氮作用。当光照强度降低时,对光照高度依赖的光合反应会降低,从而减少ATP和NADPH的产生,而固氮作用需要光合作用所积累的能量(ATP)以及还原力(NADPH)来进行固氮,所以光强的减弱会直接或间接调控固氮作用[55-56]。
另外,温度对束毛藻的固氮调节也发挥重要作用。束毛藻在20−34 ℃的温度范围内能够正常生长,其最佳生长温度为27 ℃[1, 24, 57]。不管光照条件还是黑暗条件,束毛藻固氮酶的酶活性都会随着温度的升高而升高(15−35 ℃)[58]。Rivero‐Calle等[59]认为束毛藻的固氮作用与温度之间的相关性是间接的,与其生活的暖水环境有关,较高的温度更有利于固氮,因为温度和氧气在海水中的溶解度之间成反比关系,并且在较高温度下呼吸速率更高,更有利于固氮。
5.3 二氧化碳对固氮酶活性的调节研究表明,束毛藻会根据胞内不同代谢途径的需要,将光合作用和呼吸作用中产生的能量进行分配,其中用于卡尔文循环中CO2固定所需的ATP以及还原力占据了最大的份额,其他的能量主要用于固氮以及吸收无机碳[60]。固氮所消耗的能量可能占据了每日碳固定的80%[41]。在束毛藻中,CO2和HCO3−的获取主要依靠CO2浓缩机制(carbon concentrating mechanism, CCM)的运作,CO2供应量的变化会改变CCM机制所消耗的能量[60]。当CO2分压(pCO2)升高时,节省了CCM浓缩CO2所消耗的能量,更多的能量将会流向固氮作用,从而提高了N2的固定以及颗粒有机氮(particle organic nitrogen, PON)的生产[60-62]。
5.4 不同氮源对固氮酶活性的调节束毛藻除了自身能够固定N2外,还能吸收各种形式的结合氮(NH4+、NO3−、尿素、氨基酸和DON)[63-64]。Nelson等[65]研究表明,在光周期内向束毛藻的培养基中加入铵态氮、硝态氮或尿素时,对固氮酶的合成会有所抑制。因为氮固定和氮吸收(尤其是NO3−)这两个过程都是耗能过程,二者之间对能量和还原剂具有竞争关系[65-67]。另有研究表明,向培养基中加入谷氨酸和谷氨酰胺,束毛藻固定氮的量会随着时间推移有所减少,这可能是由于代谢物池对固氮酶的反馈抑制作用[40, 66]。只有当环境中长时间非大量存在这些结合氮源时,束毛藻才能进行N2固定[68]。
5.5 铁和磷对固氮酶活性的调节在寡营养水域,水体中痕量金属元素铁的缺乏会限制束毛藻的正常生理代谢活动[69-71]。有研究通过分析束毛藻铁配额和风尘通量,发现其固氮作用在全球75%的海洋中都受到铁的限制[47-48]。因为固氮酶复合物由钼铁蛋白和铁蛋白组成,铁是构成束毛藻固氮酶复合物的重要金属辅因子,因此除了光合作用和呼吸作用过程中需要铁之外,固氮作用对铁还有额外的需求[72-73]。在自然环境中,束毛藻开发了独特的铁获取策略,包括使用富含铁的灰尘。束毛藻会捕获灰尘中的颗粒态铁并供给菌落所用,增强了其在缺铁的寡营养海域生存与竞争的能力[74-76]。这与微量金属铁的限制相似,寡营养海区缺乏有效的磷酸盐也会抑制束毛藻生长和N2固定[77-79]。磷元素是细胞中ATP、核酸生物合成、基因表达调控和翻译后修饰等过程所必需的[80],环境水体中可利用磷浓度影响束毛藻的固氮过程。
6 总结与展望固氮蓝细菌在海洋生态系统中发挥着重要的作用,为海洋中的氮循环贡献了大量新氮。其中,对海洋生物固氮贡献最大的蓝细菌束毛藻在海洋生态系统中的作用十分重要,它们形态特征、固氮方式以及与环境互作的独特性和重要性,让束毛藻成为海洋氮循环研究领域不可或缺的重要环节。束毛藻不同于其他固氮蓝细菌的不完全“时空分离”机制,可能是它们成为统治性分布的固氮蓝细菌的重要原因之一,这种将生物固氮和光合放氧进行精密调控的策略很可能是在长期进化过程中压力选择和环境适应的结果。
目前,关于束毛藻生物固氮仍有一些关键科学问题有待在未来的研究中进一步探索和深入。(1) 关于束毛藻不完全的“时空分离”策略,只是暂时将固氮作用与光合作用隔开,氧气释放对固氮酶的威胁始终存在,束毛藻作为原核生物没有细胞器(叶绿体和线粒体)的分化,氧气如何进入有氧呼吸进行能量代谢,避免对固氮系统的破坏有待进一步被揭示。(2) 束毛藻独特的固氮策略在其他固氮生物中是否存在,其进化起源或来源尚不清楚,该策略与环境适应和压力选择之间的关系还有待明确。(3) 在束毛藻的固氮调节中,单个环境因素对束毛藻固氮的调控已有大量的研究,但能量分配和平衡需要根据温度、光、铁限制及昼夜节律之间的相互作用进行复杂的调控,深入了解束毛藻的固氮调节,需要综合考虑多个环境因子的共同作用。(4) 在全球变化背景下,束毛藻的生物固氮以及其驱动的碳氮循环广泛受到大环境生态因子(如全球变暖、海洋酸化等)的影响,这需要将实验室的生理学研究与海洋航次的大规模调查、数学模型等研究策略相结合,才能更全面地揭示海洋固氮微生物在海洋生态系统中的贡献与未来变化趋势。
| [1] |
JIANG HB, FU FX, RIVERO-CALLE S, LEVINE NM, SAÑUDO-WILHELMY SA, QU PP, WANG XW, PINEDO-GONZALEZ P, ZHU Z, HUTCHINS DA. Ocean warming alleviates iron limitation of marine nitrogen fixation[J]. Nature Climate Change, 2018, 8: 709-712. DOI:10.1038/s41558-018-0216-8 |
| [2] |
ZEHR JP. Nitrogen fixation by marine cyanobacteria[J]. Trends in Microbiology, 2011, 19(4): 162-173. DOI:10.1016/j.tim.2010.12.004 |
| [3] |
WALWORTH NG, FU FX, LEE MD, CAI XN, SAITO MA, WEBB EA, HUTCHINS DA. Nutrient-colimited Trichodesmium as a nitrogen source or sink in a future ocean[J]. Applied and Environmental Microbiology, 2018, 84(3): e02137-17. |
| [4] |
HUGO B, SOPHIE B, OLIVIER G, VÉRONIQUE C, AUDE B. Transfer of diazotroph-derived nitrogen towards non-diazotrophic planktonic communities: a comparative study between Trichodesmium erythraeum, Crocosphaera watsonii and Cyanothece sp.[J]. Biogeosciences, 2016, 13(13): 4005-4021. DOI:10.5194/bg-13-4005-2016 |
| [5] |
GARDNER JJ, BOYLE NR. The use of genome-scale metabolic network reconstruction to predict fluxes and equilibrium composition of N-fixing versus C-fixing cells in a diazotrophic Cyanobacterium, Trichodesmium erythraeum[J]. BMC Systems Biology, 2017, 11(1): 4. DOI:10.1186/s12918-016-0383-z |
| [6] |
CAI XN, GAO KS. Levels of daily light doses under changed day-night cycles regulate temporal segregation of photosynthesis and N2 fixation in the cyanobacterium Trichodesmium erythraeum IMS101[J]. PLoS One, 2015, 10(8): e0135401. DOI:10.1371/journal.pone.0135401 |
| [7] |
MOORE CM, MILLS MM, ARRIGO KR, BERMAN-FRANK I, BOPP L, BOYD PW, GALBRAITH ED, GEIDER RJ, GUIEU C, JACCARD SL, JICKELLS TD, ROCHE JL, LENTON TM, MAHOWALD NM, MARAÑÓN E, MARINOV I, MOORE JK, NAKATSUKA T, OSCHLIES A, SAITO MA, et al. Processes and patterns of oceanic nutrient limitation[J]. Nature Geoscience, 2013, 6(9): 701-710. DOI:10.1038/ngeo1765 |
| [8] |
JIANG HB, LOU WJ, KE WT, SONG WY, PRICE NM, QIU BS. New insights into iron acquisition by cyanobacteria: an essential role for ExbB-ExbD complex in inorganic iron uptake[J]. The ISME Journal, 2015, 9(2): 297-309. DOI:10.1038/ismej.2014.123 |
| [9] |
JIANG HB, LU XH, DENG B, LIU LM, QIU BS. Adaptive mechanisms of the model photosynthetic organisms, cyanobacteria, to iron deficiency[M]//Microbial Photosynthesis. Singapore: Springer, 2020: 197-244.
|
| [10] |
ZHANG XN, WARD BB, SIGMAN DM. Global nitrogen cycle: critical enzymes, organisms, and processes for nitrogen budgets and dynamics[J]. Chemical Reviews, 2020, 120(12): 5308-5351. DOI:10.1021/acs.chemrev.9b00613 |
| [11] |
CAFFIN M, MOUTIN T, FOSTER RA, BOURUET- AUBERTOT P, DOGLIOLI AM, BERTHELOT H, GUIEU C, GROSSO O, HELIAS-NUNIGE S, LEBLOND N, GIMENEZ A, PETRENKO AA, VERNEIL AD, BONNET S. N2 fixation as a dominant new N source in the western tropical South Pacific Ocean (OUTPACE cruise)[J]. Biogeosciences, 2018, 15(8): 2565-2585. DOI:10.5194/bg-15-2565-2018 |
| [12] |
JICKELLS TD, BUITENHUIS E, ALTIERI K, BAKER AR, CAPONE D, DUCE RA, DENTENER F, FENNEL K, KANAKIDOU M, LAROCHE J, LEE K, LISS P, MIDDELBURG JJ, MOORE JK, OKIN G, OSCHLIES A, SARIN M, SEITZINGER S, SHARPLES J, SINGH A, et al. A reevaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean[J]. Global Biogeochemical Cycles, 2017, 31(2): 289-305. |
| [13] |
FU FX, HUTCHINS DA, JIANG HB, GAO KS. Nitrogen fixation determination method[M]//GAO KS. Research Methods in The Physiology of Aquatic Environments. Beijing: Science Press, 2018: 188-201 (in Chinese). 傅飞雪, HUTCHINS DA, 姜海波, 高坤山. 氮固定测定方法[M]//高坤山. 水域环境生理学研究方法. 北京: 科学出版社, 2018: 188-201. |
| [14] |
GRUBER N. Consistent patterns of nitrogen fixation identified in the ocean[J]. Nature, 2019, 566(7743): 191-193. DOI:10.1038/d41586-019-00498-y |
| [15] |
TANG WY, WANG S, FONSECA-BATISTA D, DEHAIRS F, GIFFORD S, GONZALEZ AG, GALLINARI M, PLANQUETTE H, SARTHOU G, CASSAR N. Revisiting the distribution of oceanic N2 fixation and estimating diazotrophic contribution to marine production[J]. Nature Communications, 2019, 10: 831. DOI:10.1038/s41467-019-08640-0 |
| [16] |
HUTCHINS DA, CAPONE DG. The marine nitrogen cycle: new developments and global change[J]. Nature Reviews Microbiology, 2022, 20(7): 401-414. DOI:10.1038/s41579-022-00687-z |
| [17] |
WANG WL, MOORE JK, MARTINY AC, PRIMEAU FW. Convergent estimates of marine nitrogen fixation[J]. Nature, 2019, 566(7743): 205-211. DOI:10.1038/s41586-019-0911-2 |
| [18] |
GOLDBERG I, NADLER V, HOCHMAN A. Mechanism of nitrogenase switch-off by oxygen[J]. Journal of Bacteriology, 1987, 169(2): 874-879. DOI:10.1128/jb.169.2.874-879.1987 |
| [19] |
YANG NN, MERKEL CA, LIN YA, LEVINE NM, HAWCO NJ, JIANG HB, QU PP, DEMERS MA, WEBB EA, FU FX, HUTCHINS DA. Warming iron-limited oceans enhance nitrogen fixation and drive biogeographic specialization of the globally important Cyanobacterium Crocosphaera[J]. Frontiers in Marine Science, 2021, 8: 628363. DOI:10.3389/fmars.2021.628363 |
| [20] |
SCHVARCZ CR, WILSON ST, CAFFIN M, STANCHEVA R, LI Q, TURK-KUBO KA, WHITE AE, KARL DM, ZEHR JP, STEWARD GF. Overlooked and widespread pennate diatom-diazotroph symbioses in the sea[J]. Nature Communications, 2022, 13: 799. DOI:10.1038/s41467-022-28065-6 |
| [21] |
ZEHR JP, CAPONE DG. Changing perspectives in marine nitrogen fixation[J]. Science, 2020, 368(6492): eaay9514. DOI:10.1126/science.aay9514 |
| [22] |
DELMONT TO, PIERELLA KARLUSICH JJ, VESELI I, FUESSEL J, EREN AM, FOSTER RA, BOWLER C, WINCKER P, PELLETIER E. Heterotrophic bacterial diazotrophs are more abundant than their cyanobacterial counterparts in metagenomes covering most of the sunlit ocean[J]. The ISME Journal, 2022, 16(4): 927-936. DOI:10.1038/s41396-021-01135-1 |
| [23] |
GRADOVILLE MR, FARNELID H, WHITE AE, TURK-KUBO KA, STEWART B, RIBALET F, FERRÓN S, PINEDO-GONZALEZ P, ARMBRUST EV, KARL DM, JOHN S, ZEHR JP. Latitudinal constraints on the abundance and activity of the cyanobacterium UCYN-A and other marine diazotrophs in the North Pacific[J]. Limnology and Oceanography, 2020, 65(8): 1858-1875. DOI:10.1002/lno.11423 |
| [24] |
CAPONE DG, ZEHR JP, PAERL HW, BERGMAN B, CARPENTER EJ. Trichodesmium, a globally significant marine cyanobacterium[J]. Science, 1997, 276(5316): 1221-1229. DOI:10.1126/science.276.5316.1221 |
| [25] |
CAPONE DG. Coming full circle on diazotrophy in the marine cyanobacterium Trichodesmium[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(47): e2117967118. |
| [26] |
BOATMAN TG, OXBOROUGH K, GLEDHILL M, LAWSON T, GEIDER RJ. An integrated response of Trichodesmium erythraeum IMS101 growth and photo-physiology to iron, CO2, and light intensity[J]. Frontiers in Microbiology, 2018, 9: 624. DOI:10.3389/fmicb.2018.00624 |
| [27] |
ZHANG YY, DONG JD, WANG HK, WANG YS, ZHANG (C/S), HUANG LM. Research development of marine cyanobacteria Trichodesmium spp.[J]. Marine Sciences, 2007, 31(3): 84-88. (in Chinese) 张燕英, 董俊德, 王汉奎, 王友绍, 张偲, 黄良民. 海洋蓝藻束毛藻的研究进展[J]. 海洋科学, 2007, 31(3): 84-88. DOI:10.3969/j.issn.1000-3096.2007.03.018 |
| [28] |
BAR-ZEEV E, AVISHAY I, BIDLE KD, BERMAN-FRANK I. Programmed cell death in the marine cyanobacterium Trichodesmium mediates carbon and nitrogen export[J]. The ISME Journal, 2013, 7(12): 2340-2348. DOI:10.1038/ismej.2013.121 |
| [29] |
DEUTSCH C, SARMIENTO JL, SIGMAN DM, GRUBER N, DUNNE JP. Spatial coupling of nitrogen inputs and losses in the ocean[J]. Nature, 2007, 445(7124): 163-167. DOI:10.1038/nature05392 |
| [30] |
SOHM JA, WEBB EA, CAPONE DG. Emerging patterns of marine nitrogen fixation[J]. Nature Reviews Microbiology, 2011, 9(7): 499-508. DOI:10.1038/nrmicro2594 |
| [31] |
SEEFELDT LC, YANG ZY, LUKOYANOV DA, HARRIS DF, DEAN DR, RAUGEI S, HOFFMAN BM. Reduction of substrates by nitrogenases[J]. Chemical Reviews, 2020, 120(12): 5082-5106. DOI:10.1021/acs.chemrev.9b00556 |
| [32] |
BURÉN S, JIMÉNEZ-VICENTE E, ECHAVARRI- ERASUN C, RUBIO LM. Biosynthesis of nitrogenase cofactors[J]. Chemical Reviews, 2020, 120(12): 4921-4968. DOI:10.1021/acs.chemrev.9b00489 |
| [33] |
CHEN Z, JIANG HB, GAO KS, QIU BS. Acclimation to low ultraviolet-B radiation increases photosystem I abundance and cyclic electron transfer with enhanced photosynthesis and growth in the cyanobacterium Nostoc sphaeroides[J]. Environmental Microbiology, 2020, 22(1): 183-197. DOI:10.1111/1462-2920.14836 |
| [34] |
SHANG JL, CHEN M, HOU SW, LI T, YANG YW, LI Q, JIANG HB, DAI GZ, ZHANG ZC, HESS WR, QIU BS. Genomic and transcriptomic insights into the survival of the subaerial cyanobacterium Nostoc flagelliforme in arid and exposed habitats[J]. Environmental Microbiology, 2019, 21(2): 845-863. DOI:10.1111/1462-2920.14521 |
| [35] |
XU Y, ZHANG JC, WANG GL, ZHUANG JY. Advance of study on nitrogenase[J]. Journal of Biology, 2011, 28(4): 61-64. (in Chinese) 徐晔, 张金池, 王广林, 庄家尧. 固氮酶的研究进展[J]. 生物学杂志, 2011, 28(4): 61-64. DOI:10.3969/j.issn.2095-1736.2011.04.061 |
| [36] |
HELD NA, WATERBURY JB, WEBB EA, KELLOGG RM, McILVIN MR, JAKUBA M, VALOIS FW, MORAN DM, SUTHERLAND KM, SAITO MA. Dynamic diel proteome and daytime nitrogenase activity supports buoyancy in the cyanobacterium Trichodesmium[J]. Nature Microbiology, 2022, 7(2): 300-311. DOI:10.1038/s41564-021-01028-1 |
| [37] |
BERMAN-FRANK I, QUIGG A, FINKEL ZV, IRWIN AJ, HARAMATY L. Nitrogen-fixation strategies and Fe requirements in cyanobacteria[J]. Limnology and Oceanography, 2007, 52(5): 2260-2269. DOI:10.4319/lo.2007.52.5.2260 |
| [38] |
JENSEN BB, COX RP. Effect of oxygen concentration on dark nitrogen fixation and respiration in cyanobacteria[J]. Archives of Microbiology, 1983, 135(4): 287-292. DOI:10.1007/BF00413483 |
| [39] |
INOMURA K, DEUTSCH C, WILSON ST, MASUDA T, LAWRENZ E, BUČINSKÁ L, SOBOTKA R, GAUGLITZ JM, SAITO MA, PRÁŠIL O, FOLLOWS MJ. Quantifying oxygen management and temperature and light dependencies of nitrogen fixation by Crocosphaera watsonii[J]. mSphere, 2019, 4(6): e00531-19. |
| [40] |
BERGMAN B, SANDH G, LIN SJ, LARSSON J, CARPENTER EJ. Trichodesmium - a widespread marine cyanobacterium with unusual nitrogen fixation properties[J]. FEMS Microbiology Reviews, 2013, 37(3): 286-302. DOI:10.1111/j.1574-6976.2012.00352.x |
| [41] |
INOMURA K, WILSON ST, DEUTSCH C. Mechanistic model for the coexistence of nitrogen fixation and photosynthesis in marine Trichodesmium[J]. mSystems, 2019, 4(4): e00210-19. |
| [42] |
CARPENTER EJ, PRICE CC. Nitrogen fixation, distribution, and production of Oscillatoria (Trichodesmium) spp. in the western Sargasso and Caribbean Seas[J]. Limnology and Oceanography, 1977, 22(1): 60-72. DOI:10.4319/lo.1977.22.1.0060 |
| [43] |
GALLON JR. Reconciling the incompatible: N2 fixation and O2[J]. New Phytologist, 1992, 122(4): 571-609. DOI:10.1111/j.1469-8137.1992.tb00087.x |
| [44] |
EL-SHEHAWY R, LUGOMELA C, ERNST A, BERGMAN B. Diurnal expression of hetR and diazocyte development in the filamentous non-heterocystous cyanobacterium Trichodesmium erythraeum[J]. Microbiology, 2003, 149(5): 1139-1146. DOI:10.1099/mic.0.26170-0 |
| [45] |
CORNEJO-CASTILLO FM, ZEHR JP. Hopanoid lipids may facilitate aerobic nitrogen fixation in the ocean[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(37): 18269-18271. |
| [46] |
MULHOLLAND MR, CAPONE DG. The nitrogen physiology of the marine N2-fixing cyanobacteria Trichodesmium spp.[J]. Trends in Plant Science, 2000, 5(4): 148-153. DOI:10.1016/S1360-1385(00)01576-4 |
| [47] |
BERMAN-FRANK I, LUNDGREN P, CHEN YB, KÜPPER H, KOLBER Z, BERGMAN B, FALKOWSKI P. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium[J]. Science, 2001, 294(5546): 1534-1537. DOI:10.1126/science.1064082 |
| [48] |
BOATMAN TG, DAVEY PA, LAWSON T, GEIDER RJ. CO2 modulation of the rates of photosynthesis and light-dependent O2 consumption in Trichodesmium[J]. Journal of Experimental Botany, 2019, 70(2): 589-597. DOI:10.1093/jxb/ery368 |
| [49] |
FINZI-HART JA, PETT-RIDGE J, WEBER PK, POPA R, FALLON SJ, GUNDERSON T, HUTCHEON ID, NEALSON KH, CAPONE DG. Fixation and fate of C and N in the cyanobacterium Trichodesmium using nanometer-scale secondary ion mass spectrometry[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(15): 6345-6350. |
| [50] |
EICHNER M, THOMS S, ROST B, MOHR W, AHMERKAMP S, PLOUG H, KUYPERS MMM, BEER DD. N2 fixation in free-floating filaments of Trichodesmium is higher than in transiently suboxic colony microenvironments[J]. New Phytologist, 2019, 222(2): 852-863. DOI:10.1111/nph.15621 |
| [51] |
AGARWAL V, INOMURA K, MOUW CB. Quantitative analysis of the trade-offs of colony formation for Trichodesmium[J]. Microbiology Spectrum, 2022, 10(6): e02025-22. |
| [52] |
GARDNER JJ, HODGE BMS, BOYLE NR. Investigating the unique ability of Trichodesmium to fix carbon and nitrogen simultaneously using MiMoSA[J]. mSystems, 2023, 8(1): e00601-20. |
| [53] |
CHANG YG, COHEN SE, PHONG C, MYERS WK, KIM YI, TSENG R, LIN J, ZHANG L, BOYD JS, LEE Y, KANG S, LEE D, LI S, BRITT RD, RUST MJ, GOLDEN SS, LiWANG A. A protein fold switch joins the circadian oscillator to clock output in cyanobacteria[J]. Science, 2015, 349(6245): 324-328. DOI:10.1126/science.1260031 |
| [54] |
TURK KA, REES AP, ZEHR JP, PEREIRA N, SWIFT P, SHELLEY R, LOHAN M, WOODWARD EMS, GILBERT J. Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic[J]. The ISME Journal, 2011, 5(7): 1201-1212. DOI:10.1038/ismej.2010.205 |
| [55] |
LU YY, WEN ZZ, SHI DL, CHEN MM, ZHANG Y, BONNET S, LI YH, TIAN JW, KAO SJ. Effect of light on N2 fixation and net nitrogen release of Trichodesmium in a field study[J]. Biogeosciences, 2018, 15(1): 1-12. DOI:10.5194/bg-15-1-2018 |
| [56] |
YI XQ, FU FX, HUTCHINS DA, GAO KS. Light availability modulates the effects of warming in a marine N2 fixer[J]. Biogeosciences, 2020, 17(4): 1169-1180. DOI:10.5194/bg-17-1169-2020 |
| [57] |
DING CL, SUN J, XUE B. Winter and summer diazotrophic cyanobacteria in the western South China Sea[J]. Acta Oceanologica Sinica, 2016, 38(4): 84-94. (in Chinese) 丁昌玲, 孙军, 薛冰. 冬夏季南海西部固氮蓝藻[J]. 海洋学报, 2016, 38(4): 84-94. DOI:10.3969/j.issn.0253-4193.2016.04.008 |
| [58] |
STAAL M, MEYSMAN FJR, STAL LJ. Temperature excludes N2-fixing heterocystous cyanobacteria in the tropical oceans[J]. Nature, 2003, 425(6957): 504-507. DOI:10.1038/nature01999 |
| [59] |
RIVERO-CALLE S, del CASTILLO CE, GNANADESIKAN A, DEZFULI A, ZAITCHIK B, JOHNS DG. Interdecadal Trichodesmium variability in cold North Atlantic waters[J]. Global Biogeochemical Cycles, 2016, 30(11): 1620-1638. DOI:10.1002/2015GB005361 |
| [60] |
KRANZ SA, EICHNER M, ROST B. Interactions between CCM and N2 fixation in Trichodesmium[J]. Photosynthesis Research, 2011, 109(1): 73-84. |
| [61] |
RAMOS JBE, BISWAS H, SCHULZ KG, LaROCHE J, RIEBESELL U. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium[J]. Global Biogeochemical Cycles, 2007, 21(2): GB2028. |
| [62] |
HUTCHINS DA, FU FX, WEBB EA, WALWORTH N, TAGLIABUE A. Taxon-specific response of marine nitrogen fixers to elevated carbon dioxide concentrations[J]. Nature Geoscience, 2013, 6(9): 790-795. DOI:10.1038/ngeo1858 |
| [63] |
BERTHELOT H, BONNET S, CAMPS M, GROSSO O, MOUTIN T. Assessment of the dinitrogen released as ammonium and dissolved organic nitrogen by unicellular and filamentous marine diazotrophic cyanobacteria grown in culture[J]. Frontiers in Marine Science, 2015, 2: 80. |
| [64] |
POST AF, RIHTMAN B, WANG QF. Decoupling of ammonium regulation and ntcA transcription in the diazotrophic marine cyanobacterium Trichodesmium sp. IMS101[J]. The ISME Journal, 2012, 6(3): 629-637. DOI:10.1038/ismej.2011.121 |
| [65] |
NELSON JA, STALLINGS CD, LANDING WM, CHANTON J. Biomass transfer subsidizes nitrogen to offshore food webs[J]. Ecosystems, 2013, 16(6): 1130-1138. |
| [66] |
KLAWONN I, EICHNER MJ, WILSON ST, MORADI N, THAMDRUP B, KÜMMEL S, GEHRE M, KHALILI A, GROSSART HP, KARL DM, PLOUG H. Distinct nitrogen cycling and steep chemical gradients in Trichodesmium colonies[J]. The ISME Journal, 2020, 14(2): 399-412. |
| [67] |
BOATMAN TG, DAVEY PA, LAWSON T, GEIDER RJ. The physiological cost of diazotrophy for Trichodesmium erythraeum IMS101[J]. PLoS One, 2018, 13(4): e0195638. |
| [68] |
KNAPP AN. The sensitivity of marine N2 fixation to dissolved inorganic nitrogen[J]. Frontiers in Microbiology, 2012, 3: 374. |
| [69] |
ZHANG FT, WEN ZZ, WANG SL, TANG WY, LUO YW, KRANZ SA, HONG HZ, SHI DL. Phosphate limitation intensifies negative effects of ocean acidification on globally important nitrogen fixing cyanobacterium[J]. Nature Communications, 2022, 13: 6730. |
| [70] |
LEVITAN O, BROWN CM, SUDHAUS S, CAMPBELL D, LAROCHE J, BERMAN-FRANK I. Regulation of nitrogen metabolism in the marine diazotroph Trichodesmium IMS101 under varying temperatures and atmospheric CO2 concentrations[J]. Environmental Microbiology, 2010, 12(7): 1899-1912. |
| [71] |
CERDAN-GARCIA E, BAYLAY A, POLYVIOU D, WOODWARD EMS, WRIGHTSON L, MAHAFFEY C, LOHAN MC, MOORE CM, BIBBY TS, ROBIDART JC. Transcriptional responses of Trichodesmium to natural inverse gradients of Fe and P availability[J]. The ISME Journal, 2022, 16(4): 1055-1064. |
| [72] |
CHAPPELL PD, MOFFETT JW, HYNES AM, WEBB EA. Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium[J]. The ISME Journal, 2012, 6(9): 1728-1739. |
| [73] |
DETONI AMS, SUBRAMANIAM A, HALEY ST, DYHRMAN ST, CALIL PHR. Cyanobacterial diazotroph distributions in the western South Atlantic[J]. Frontiers in Marine Science, 2022, 9: 856643. |
| [74] |
KESSLER N, ARMOZA-ZVULONI R, WANG SY, BASU S, WEBER PK, STUART RK, SHAKED Y. Selective collection of iron-rich dust particles by natural Trichodesmium colonies[J]. The ISME Journal, 2020, 14(1): 91-103. |
| [75] |
BASU S, GLEDHILL M, BEER DD, MATONDKAR SGP, SHAKED Y. Colonies of marine cyanobacteria Trichodesmium interact with associated bacteria to acquire iron from dust[J]. Communications Biology, 2019, 2: 284. |
| [76] |
LIU LM, LI DL, DENG B, WANG XW, JIANG HB. Special roles for efflux systems in iron homeostasis of non-siderophore-producing cyanobacteria[J]. Environmental Microbiology, 2022, 24(2): 551-565. |
| [77] |
HELD NA, WEBB EA, McILVIN MM, HUTCHINS DA, COHEN NR, MORAN DM, KUNDE K, LOHAN MC, MAHAFFEY C, WOODWARD EMS, SAITO MA. Co-occurrence of Fe and P stress in natural populations of the marine diazotroph Trichodesmium[J]. Biogeosciences, 2020, 17(9): 2537-2551. |
| [78] |
ZHU Z, FU FX, QU PP, MAK EWK, JIANG HB, ZHANG RF, ZHU ZY, GAO KS, HUTCHINS DA. Interactions between ultraviolet radiation exposure and phosphorus limitation in the marine nitrogen-fixing cyanobacteria Trichodesmium and Crocosphaera[J]. Limnology and Oceanography, 2020, 65(2): 363-376. |
| [79] |
LI XF, FONSECA-BATISTA D, ROEVROS N, DEHAIRS F, CHOU L. Environmental and nutrient controls of marine nitrogen fixation[J]. Progress in Oceanography, 2018, 167: 125-137. |
| [80] |
LI JH, WANG ZW, CAO X, WANG ZF, ZHENG Z. Effect of orthophosphate and bioavailability of dissolved organic phosphorous compounds to typically harmful cyanobacterium Microcystis aeruginosa[J]. Marine Pollution Bulletin, 2015, 92(1/2): 52-58. |
 2023, Vol. 50
2023, Vol. 50