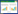扩展功能
文章信息
- 刘鹏鸿, 张克让
- LIU Penghong, ZHANG Kerang
- 首发重性抑郁症患者肠道菌群组成及与胃肠道症状的相关性
- Gut microbiota in patients with first-episode major depressive disorder: composition and correlations with gastrointestinal symptoms
- 微生物学通报, 2023, 50(8): 3575-3587
- Microbiology China, 2023, 50(8): 3575-3587
- DOI: 10.13344/j.microbiol.china.230462
-
文章历史
- 收稿日期: 2023-06-07
- 接受日期: 2023-06-23
- 网络首发日期: 2023-07-19
2. 山西医科大学第一临床医学院, 山西 太原 030001
2. The First Clinical Medical College of Shanxi Medical University, Taiyuan 030001, Shanxi, China
重性抑郁症(major depressive disorder, MDD)是最常见的精神疾病之一,在中国MDD的发病率为3.4%[1]。除情感和认知症状外,MDD患者常常伴有胃肠道症状,如食欲不振、腹痛、腹胀和便秘等,且胃肠道症状与焦虑和抑郁的严重程度密切相关[2]。研究发现超过70%的MDD患者伴有胃肠道症状,并且胃肠道症状的发作频率与自杀意念、自杀行为、焦虑、抑郁、失眠和易激惹的风险增加呈正相关[3]。同样地,胃肠道疾病患者更容易出现抑郁症状[4]。一项临床研究发现,抑郁症在肠易激综合征(irritable bowel syndrome, IBS)和腹痛患者中更常见[5]。由此可见,抑郁症状与胃肠道症状密切相关。有证据表明,抑郁症患者的抑郁和胃肠道症状可能具有共同的病理生理机制,其机制可能涉及神经内分泌、神经免疫、肠道菌群和神经可塑性等[6]。
近年来,越来越多的证据表明,肠道微生物在精神障碍的发生和发展中发挥着重要作用[7-8]。肠道菌群的改变被推测为MDD的潜在病因,可以通过“肠-脑轴”影响宿主的脑功能和行为[9-10]。一个重要机制是肠道菌群失调可能会激活外周和中枢炎症反应[11]。同样地,肠道菌群紊乱被认为与许多胃肠道疾病和胃肠道症状有关[4]。然而,目前尚无研究探索MDD患者中失调肠道微生物是否与激活的炎症反应和胃肠道症状相关。
本研究探讨了首发未治疗的MDD患者肠道菌群的特征,进一步分析了改变的肠道菌群是否与炎症标志物和胃肠道症状相关,以期为基于微生物组的MDD患者的抑郁症状和胃肠道症状治疗提供了依据。
1 材料与方法 1.1 材料募集2019年12月−2022年4月在山西医科大学第一医院精神卫生科就诊的符合入排标准的MDD受试者91例。
入组标准:(1) 符合美国精神障碍诊断与统计手册第4版(Diagnostic and Statistical Manual of Mental Disorders, fourth edition, DSM-IV) 重性抑郁障碍诊断标准;(2) 首发未治疗;(3) 18岁≤年龄≤50岁;(4) 汉密尔顿抑郁量表17项(Hamilton depression scale-17, HAMD-17)总分 > 17分[12]。
排除标准:(1) 患有其他精神疾病;(2) 患有其他躯体疾病,尤其胃肠道疾病;(3) 有严重自杀倾向者;(4) 有酒或其他物质依赖或滥用证据者;(5) 家族史:双相障碍、精神分裂症等精神疾病家族史;(6) 治疗史:最近1个月内接受过抗生素、益生菌、免疫抑制剂等治疗;(7) 生育史:妊娠或哺乳期女性;(8) 在过去90 d内已经参加过一项临床试验或在过去1年内参加过2项及以上临床试验。
通过在社区张贴招募广告招募健康对照者(healthy controls, HCs) 105名。排除标准同MDD组。两组参与者在参与试验前均签署了书面知情同意书。本研究获得山西医科大学第一医院研究伦理委员会批准(K-K004)。
OMEGA Soil DNA Kit,OMEGA Bio-Tek公司;高敏C反应蛋白(high-sensitivity C-reactive protein, hs-CRP)、白细胞介素-1β (interleukin-1β, IL-1β)、白细胞介素-6 (interleukin-6, IL-6)、白细胞介素-10 (interleukin-10, IL-10)和肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)的酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)检测试剂盒,江莱生物科技有限公司。酶标分析仪,Rayto公司;NanoDrop One分光光度计,ThermoFisher公司。
1.2 胃肠道症状评估采用胃肠症状评定量表(gastrointestinal symptom rating scale, GSRS)评估胃肠道症状的严重程度。GSRS常用于评估胃肠道疾病的常见症状[13]。包含15个条目,每个条目采用0−3分的4级分法,相应分数对应的症状程度为:0为无症状;1为轻度,偶发的症状;2为中度,频发的症状;3为重度,症状持续存在。该研究中我们对GSRS做了一些小的修改,即去除原量表中的第4项上腹部紧抽感,增加了食欲下降和早饱感2项,使其更适合评估MDD的胃肠道症状。
1.3 粪便和血液样本收集收集受试者粪便样本2 mg,并立即冷冻至−80 ℃冰箱,用于肠道微生物检测。于清晨6:30−7:30采集空腹肘静脉血10 mL,置于10 mL抗凝管中,以3 500 r/min的转速离心10 min分离得到血浆,放于−80 ℃冰箱保存,通过ELISA检测炎症因子hs-CRP、IL-1β、IL-6、IL-10和TNF-α。
1.4 粪便样本DNA提取、16S rRNA基因测序及生物信息学数据分析按照说明书使用OMEGA Soil DNA Kit提取总基因组DNA样本,并在−20 ℃下保存。然后使用NanoDrop One分光光度计定量DNA,并使用0.8%琼脂糖凝胶电泳测量DNA样品的完整性和大小。以细菌16S rRNA基因的V3−V4可变区序列为靶标,以338F (5′-ACTCCTACGG GAGGCAGCA-3′)和806R (5′-GGACTACHVGG GTWTCTAAT-3′)为引物,使用Illumina NovaSeq PE250进行PCR扩增和测序文库的制备。PCR反应体系(25 μL):5×buffer 5 μL,dNTPs (2.5 mmol/L) 2 μL,上、下游引物(10 μmol/L)各1 μL,DNA模板1 μL,ddH2O 14.75 μL,FastPfu DNA polymerase (5 U/μL) 0.25 μL。PCR反应条件:98 ℃ 5 min;98 ℃ 30 s,53 ℃ 30 s,72 ℃ 45 s,25个循环;72 ℃ 5 min。
采用QIIME 2软件(V2019.1)[14]对测序序列进行质控及操作分类单元(operational taxonomic unit, OTU)聚类,OTU代表序列与Greengenes数据库进行比对分析,获取OTU对应的分类单元(包括门纲目科属种)及其相应的丰度信息。
使用QIIME 2 (V2019.1)计算α和β多样性指标。α多样性指数分别为Shannon指数、Simpson指数、observed species指数、Chao1指数、Faith指数、Pielous均匀度指数和Goods覆盖度指数。采用基于Jaccard非相似度的非度量多维尺度(non-metric multidimensional scaling, NMDS)方法进行β多样性分析。采用线性判别分析(linear discriminant analysis effect size, LEfSe)鉴定MDD和HCs之间差异菌属,使用Spearman相关系数构建差异菌属、细胞因子、抑郁症状和胃肠道症状之间的相关矩阵。使用GenesCloud程序(https://www.genescloud.cn/chart/CorHeatmap)生成相关矩阵的热图。
1.5 统计分析所有分析均使用SPSS23软件完成。采用独立样本t检验分析两组间年龄、受教育年限、HAMD-17总分和胃肠道症状分数差异。采用卡方检验比较两组间性别差异。P < 0.05 (双尾)表示差异有统计学意义。采用Pearson或Spearman方法对差异肠道菌属、炎性因子、临床症状进行相关分析。
2 结果与分析 2.1 一般人口学资料、炎性指标及临床症状两组受试者之间年龄、性别、体重指数差异无统计学意义,但MDD患者的平均受教育年限明显少于HCs (P < 0.001)。MDD组的HAMD-17和GSRS总分明显高于HCs组(P < 0.001)。MDD患者外周血hs-CRP水平也明显高于HCs (P < 0.001),但两组间IL-1β、IL-6、IL-10和TNF-α差异无统计学意义(P > 0.05) (表 1)。
| Basic information | MDD (n=91) | HCs (n=105) | t/χ2 | P |
| Gender, male/female | 57/34 | 63/42 | 0.143 | 0.705 a |
| Age (years) | 22.30±7.04 | 23.74±3.31 | 1.880 | 0.062 b |
| Level of education completed (years) | 12.86±3.45 | 16.70±2.08 | 9.558 | 0.001 b* |
| Body mass index | 21.17±3.22 | 21.72±3.38 | 1.173 | 0.242 b |
| HAMD-17 | 24.80±6.18 | 2.21±3.98 | 33.817 | 0.001 b* |
| GSRS | 9.40±6.29 | 1.58±2.34 | 11.832 | 0.001 b* |
| hs-CRP (ng/mL) | 131.75±22.96 | 99.50±21.96 | 9.550 | 0.001 b* |
| IL-1β (pg/mL) | 183.89±39.34 | 179.44±36.15 | 0.784 | 0.434 b |
| IL-6 (pg/mL) | 119.24±27.09 | 120.35±24.76 | 0.285 | 0.776 b |
| IL-10 (pg/mL) | 160.17±27.52 | 161.25±29.11 | 0.254 | 0.800 b |
| TNF-α (pg/mL) | 473.25±114.78 | 486.12±102.14 | 0.788 | 0.432 b |
| a:卡方检验;b:两样本t检验;*:有统计学差异 a: P value for chi-square test; b: P values for two-sample t-test; *: Significant difference. |
||||
所有的MDD患者都伴有胃肠道症状,在16个胃肠道症状中,排名前5的有食欲下降、早饱感、恶心呕吐、排便不畅感和腹胀,其发生率分别为82.42%、74.73%、70.33%、52.75%和43.96% (表 2)。
| Item | Severity degree (number of people) | Number of people with gastrointestinal symptoms | Incidence of gastrointestinal symptoms (%) | |||
| Number | Occasional of short duration | Frequent and prolonged discomfort | Persistent severe discomfort | |||
| Abdominal pain | 56 | 28 | 7 | 0 | 35 | 38.46 |
| Abdominal distension | 51 | 28 | 7 | 5 | 40 | 43.96 |
| Heartburn | 64 | 21 | 3 | 3 | 27 | 29.67 |
| Acid regurgitation | 52 | 35 | 3 | 1 | 39 | 42.86 |
| Nausea and vomiting | 27 | 45 | 14 | 5 | 64 | 70.33 |
| Anorexia | 16 | 37 | 30 | 8 | 75 | 82.42 |
| Early satiation | 33 | 39 | 15 | 4 | 68 | 74.73 |
| Eructation | 61 | 20 | 8 | 2 | 30 | 32.97 |
| Borborygmus | 68 | 17 | 5 | 1 | 23 | 25.27 |
| Increased flatus | 55 | 28 | 7 | 1 | 36 | 39.56 |
| Decreased passage of stools | 56 | 28 | 3 | 4 | 35 | 38.46 |
| Increased passage of stools | 84 | 7 | 0 | 0 | 7 | 7.69 |
| Loose stools | 62 | 15 | 10 | 4 | 29 | 31.87 |
| Hard stools | 49 | 18 | 4 | 11 | 33 | 36.26 |
| Urgent need for defecation | 60 | 24 | 7 | 0 | 31 | 34.07 |
| Feeling of incomplete evacuation | 43 | 29 | 14 | 5 | 48 | 52.75 |
α多样性分析显示,MDD患者的Simpson指数和Pielous均匀度指数均低于HCs (P < 0.05),但两组间Shannon指数、observed_species指数、Chao1指数、Faith指数和Goods覆盖度指数差异无统计学意义(P > 0.05) (图 1)。
|
| 图 1 MDD患者和HCs之间α多样性差异 Figure 1 Difference of alpha diversity between MDD patients and HCs. *: P < 0.05. |
|
|
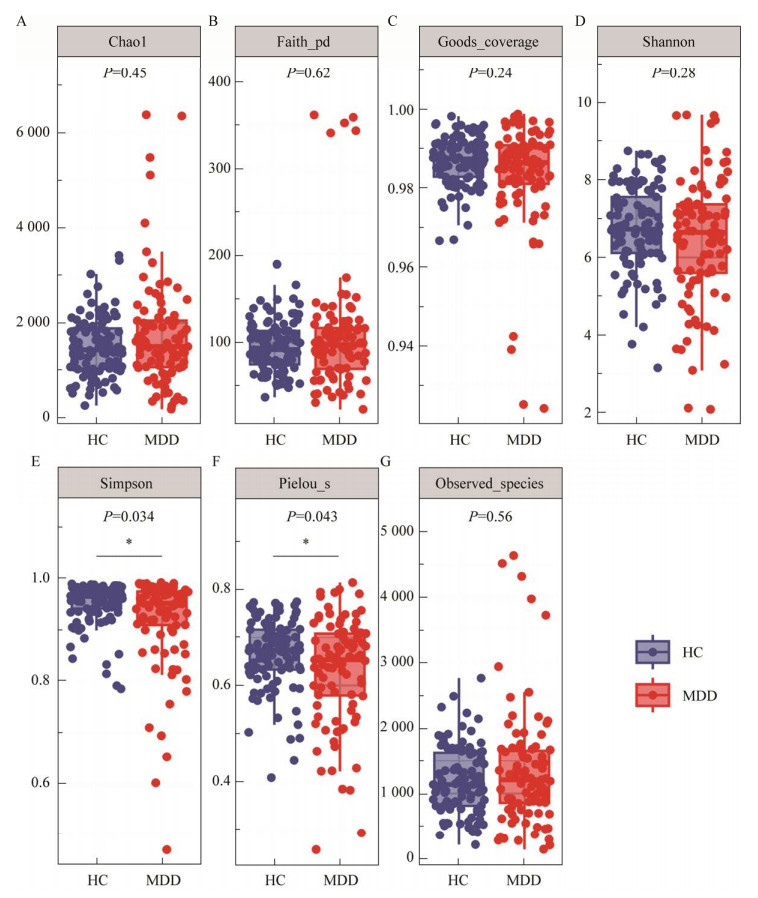
基于Jaccard非相似度的NMDS β多样性分析发现MDD患者与HCs的肠道菌群分布存在显著差异(图 2)。
|
| 图 2 MDD患者和HCs之间β多样性差异 Figure 2 Difference of beta diversity between MDD patients and HCs. |
|
|
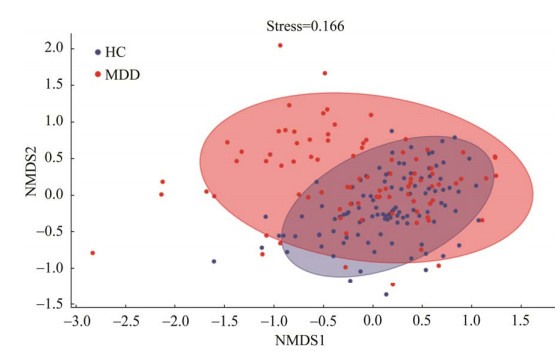
LEfSe分析发现,在属水平上,MDD组中地嗜皮菌属、异斯卡多维亚菌属、双歧杆菌属、布洛特菌属、钩丝菌属、红长菌属、马赛菌属、嗜血杆菌属、Candidatus Xiphinematobacter和Chthoniobacter相对丰度较高,而拟杆菌属、副拟杆菌属、SMB53、厌氧菌属、梭菌属、毛梭菌属、罗斯氏菌属、粪杆菌属、瘤胃球菌属、小杆菌属、考拉杆菌属和萨特氏菌属的相对丰度降低(图 3)。
|
| 图 3 MDD患者和HCs之间LEfSe分类生物标志物 Figure 3 Taxonomic biomarkers found by LEfSe in MDD patients and HCs. 横坐标的数字表示差异分类单元的LDA分析对数得分值 Horizontal coordinate numbers represent the LDA scores for significantly different abundant taxa. |
|
|
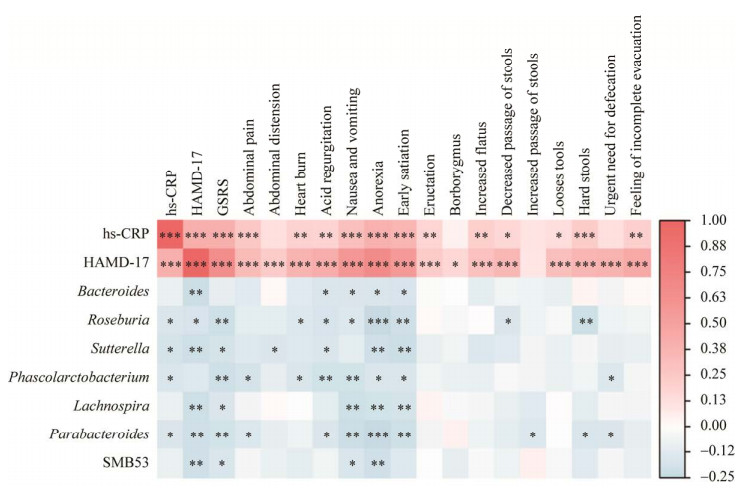
为了确定关键致病菌属,明确MDD患者的抑郁症状和胃肠道症状是否存在共同的病理生理机制,本研究进行相关分析发现罗斯氏菌属、萨特氏菌属、考拉杆菌属和副拟杆菌属的相对丰度与hs-CRP浓度呈负相关(P < 0.05)。同时,罗斯氏菌属、萨特氏菌属和副拟杆菌属的相对丰度与HAMD-17总分、GSRS总分和GSRS部分条目评分呈负相关(P < 0.05)。hs-CRP浓度与HAMD-17总分、GSRS总分和GSRS部分条目评分呈正相关(P < 0.05)。HAMD-17总分与GSRS总分和GSRS中14个条目评分呈正相关(P < 0.05) (图 4)。
|
| 图 4 MDD患者和HCs之间差异肠道菌属与hs-CRP、HAMD-17、GSRS总分及部分项目分数的Spearman相关系数矩阵热图 Figure 4 The heatmaps of Spearman correlation coefficient matrix between significant different gut bacteria genera and hs-CRP, the HAMD-17, the total score and some items of GSRS in all subjects. *: P < 0.05, **: P < 0.01, ***: P < 0.001. |
|
|
本研究发现,与HCs相比,MDD组hs-CRP浓度显著升高。抑郁症的神经免疫假说于1995年被首次提出[15]。在过去的几十年里,大量证据表明炎症与MDD的发生和发展密切相关[16-17]。MDD与促炎细胞因子增加有关,如IL-6、TNF-α和hs-CRP等[18-19]。考虑到各种炎症指标的稳定性、准确性和可用性,美国疾病控制和预防中心(Centers for Disease Control and Prevention, CDC)与美国心脏协会(American Heart Association, AHA)推荐将hs-CRP作为临床和公共卫生实践中的炎症指标[20]。因此hs-CRP是一个稳定的指标,可以反映MDD患者机体的整体炎症状态。
3.2 MDD患者肠道菌群改变肠道菌群分析发现MDD组和HCs之间的α多样性(Simpson指数和Pielous均匀度指数)和β多样性都存在差异。α多样性降低被认为与一系列慢性疾病有关[21]。Huang等[22]研究表明,MDD患者的Shannon、Chao1和ACE多样性指数降低。Liu等[23]基于Bray-Curtis dissimilarity和UniFrac distance发现MDD和HCs之间β多样性存在显著差异。然而一些研究报道了不同的结论。Jiang等[24]报道,与HCs相比,MDD患者肠道微生物群的多样性增加。在一项包括17项研究的系统综述中,Knudsen等[25]发现大多数研究未发现α多样性的显著差异;同样地,在β多样性的分析中也发现了矛盾的结果。高微生物多样性对健康有潜在的好处,但它很容易受到年龄、饮食和其他因素的影响[26]。
进一步研究发现在属的水平上,MDD组中地嗜皮菌属、双歧杆菌属、布洛特菌属、红长菌属、Candidatus Xiphinematobacter和Chthoniobacter等相对丰度较高,而拟杆菌属、副拟杆菌属、梭菌属、毛梭菌属、罗斯氏菌属、粪杆菌属、瘤胃球菌属和考拉杆菌属等相对丰度较低。一项包括了36名MDD患者与38名HCs的研究发现MDD患者中毛梭菌属、普氏粪杆菌和罗斯氏菌属的丰度降低[27]。Liu等[23]发现在属水平上,MDD组粪杆菌属、梭菌属和瘤胃球菌属的水平较低,而拟杆菌属的丰度较高。Cheung等[28]分析了6项研究,发现MDD患者Anaerostipes、布洛特菌属、梭菌属、毛梭菌属、副拟杆菌属、考拉杆菌属和链球菌属相对丰度升高,而双歧杆菌属、小杆菌属、粪杆菌属和瘤胃球菌属相对丰度降低。Sanada等[29]对10项研究进行了荟萃分析,同样表明粪杆菌属、瘤胃球菌属、双歧杆菌属和埃希氏杆菌属在MDD患者中减少,而Paraprevotella增加。总之,并非所有的发现与本研究的结果一致,一个可能的原因是肠道是一个复杂的生态系统,可能受到各种因素的影响,如年龄、遗传、饮食和区域差异[30]。此外,一些研究是横断面的,未评估抗精神病药物对肠道微生物群的影响[31]。
3.3 紊乱的肠道菌群与hs-CRP、抑郁症状和胃肠道症状的相关性进一步研究发现罗斯氏菌属、萨特氏菌属和副拟杆菌属的相对丰度与hs-CRP、HAMD-17总分、GSRS总分及GSRS部分条目呈负相关。罗斯氏菌属属于厚壁菌门梭菌纲梭菌目毛螺科[32],可以产生短链脂肪酸,如乙酸、丙酸和丁酸[33]。短链脂肪酸具有抗炎的作用[34]。萨特氏菌属属于β变形菌,革兰氏阴性菌,其与自闭症、唐氏综合征和代谢综合征有关[35]。一些由溃疡性结肠炎(ulcerative colitis, UC)患者组成的临床队列研究显示,萨特氏菌属的丰度与宿主炎症细胞因子浓度呈负相关[36-37]。副拟杆菌属为革兰氏阴性、无芽孢的专性厌氧菌[38]。副拟杆菌属可以通过抑制TNF-α、IL-6、IL-17、IL-12或干扰素-γ (interferon-γ, IFN-γ)的释放来控制先天性免疫反应[39]。此外,一些副拟杆菌具有通过抑制丝裂原活化蛋白激酶(mitogen activated protein kinases, MAPK)和核因子-κB (nuclear factor-κB, NF-κB)信号通路来缓解肠道炎症的能力[40]。综上所述,罗斯氏菌属、萨特氏菌属和副拟杆菌属水平的降低可能促进炎症反应,并进一步引起抑郁症状和胃肠道症状。
本研究首次探讨了首发未治疗的MDD患者紊乱的肠道菌群与炎性因子和胃肠道症状的关系,具有一定的创新性,但也存在局限性。首先,本研究使用的16S rRNA基因测序只能检测到属水平,所以在菌种或菌株水平上的关键微生物可能被忽视。其次,本研究使用的GSRS是经过修改的版本,虽然适合MDD患者胃肠道症状的评估,但其信度和效度未经过测评。再次,饮食对肠道微生物群的组成和功能有显著影响,但本研究未对受试者的饮食结构进行评估,这可能会对本研究的结果产生影响。
4 结论该研究表明,MDD患者表现出肠道微生物群紊乱和hs-CRP水平升高。MDD患者中改变的肠道菌群与hs-CRP水平、抑郁症状和胃肠道症状密切相关。
| [1] |
HUANG YQ, WANG Y, WANG H, LIU ZR, YU X, YAN J, YU YQ, KOU CG, XU XF, LU J, WANG ZZ, HE SL, XU YF, HE YL, LI T, GUO WJ, TIAN HJ, XU GM, XU XD, MA YJ, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study[J]. The Lancet Psychiatry, 2019, 6(3): 211-224. DOI:10.1016/S2215-0366(18)30511-X |
| [2] |
HAUG TT, MYKLETUN A, DAHL AA. Are anxiety and depression related to gastrointestinal symptoms in the general population?[J]. Scandinavian Journal of Gastroenterology, 2002, 37(3): 294-298. DOI:10.1080/003655202317284192 |
| [3] |
HUANG J, CAI Y, SU Y, ZHANG M, SHI Y, ZHU N, JIN F, PENG D, FANG Y. Gastrointestinal symptoms during depressive episodes in 3256 patients with major depressive disorders: findings from the NSSD[J]. Journal of Affective Disorders, 2021, 286: 27-32. DOI:10.1016/j.jad.2021.02.039 |
| [4] |
EUSTIS SJ, MCCALL MW, MURPHY EA, WIRTH MD. Association between gastrointestinal symptoms and depression in a representative sample of adults in the United States: findings from national health and nutrition examination survey (2005−2016)[J]. Journal of the Academy of Consultation-Liaison Psychiatry, 2022, 63(3): 268-279. DOI:10.1016/j.jaclp.2021.08.008 |
| [5] |
ROSE JD, TROUGHTON AH, HARVEY JS, SMITH PM. Depression and functional bowel disorders in gastrointestinal outpatients[J]. Gut, 1986, 27(9): 1025-1028. DOI:10.1136/gut.27.9.1025 |
| [6] |
GENG JT, YAN R, SHI JB, CHEN Y, MO ZQ, SHAO JN, WANG XY, LU Q, YAO ZJ. Altered regional homogeneity in patients with somatic depression: a resting-state fMRI study[J]. Journal of Affective Disorders, 2019, 246: 498-505. DOI:10.1016/j.jad.2018.12.066 |
| [7] |
BRUCE-KELLER AJ, SALBAUM JM, BERTHOUD HR. Harnessing gut microbes for mental health: getting from here to there[J]. Biological Psychiatry, 2018, 83(3): 214-223. DOI:10.1016/j.biopsych.2017.08.014 |
| [8] |
HU K, ZHANG TT, ZHANG K, WANG GQ. Correlation analysis of intestinal flora diversity and clinical symptoms in patients with first-episode depression[J]. Microbiology China, 2023, 50(3): 1040-1051. (in Chinese) 胡科, 张同同, 张凯, 王国强. 首发抑郁症患者肠道菌群多样性与抑郁症状的相关性分析[J]. 微生物学通报, 2023, 50(3): 1040-1051. DOI:10.13344/j.microbiol.china.220645 |
| [9] |
ZHENG P, ZENG B, ZHOU C, LIU M, FANG Z, XU X, ZENG L, CHEN J, FAN S, DU X, ZHANG X, YANG D, YANG Y, MENG H, LI W, MELGIRI ND, LICINIO J, WEI H, XIE P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism[J]. Molecular Psychiatry, 2016, 21(6): 786-796. DOI:10.1038/mp.2016.44 |
| [10] |
LIU PH, GAO MX, LIU ZF, ZHANG YY, TU HW, LEI L, WU PY, ZHANG AX, YANG CX, LI GZ, SUN N, ZHANG KR. Gut microbiome composition linked to inflammatory factors and cognitive functions in first-episode, drug-I major depressive disorder patients[J]. Frontiers in Neuroscience, 2022, 15: 800764. DOI:10.3389/fnins.2021.800764 |
| [11] |
SUNESON K, LINDAHL J, HÅRSMAR SC, SÖDERBERG G, LINDQVIST D. Inflammatory depression-mechanisms and non-pharmacological interventions[J]. International Journal of Molecular Sciences, 2021, 22(4): 1640. DOI:10.3390/ijms22041640 |
| [12] |
HAMILTON M. A rating scale for depression[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 1960, 23(1): 56-62. DOI:10.1136/jnnp.23.1.56 |
| [13] |
SVEDLUND J, SJÖDIN I, DOTEVALL G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease[J]. Digestive Diseases and Sciences, 1988, 33(2): 129-134. DOI:10.1007/BF01535722 |
| [14] |
BOLYEN E, RIDEOUT JR, DILLON MR, BOKULICH NA, ABNET CC, AL-GHALITH GA, ALEXANDER H, ALM EJ, ARUMUGAM M, ASNICAR F, BAI Y, BISANZ JE, BITTINGER K, BREJNROD A, BRISLAWN CJ, BROWN CT, CALLAHAN BJ, CARABALLO-RODRÍGUEZ AM, CHASE J, COPE EK, et al. Author correction: reproducible, interactive, scalable and extensible microbiome data science using QIIME 2[J]. Nature Biotechnology, 2019, 37(9): 1091. |
| [15] |
MAES M, VANDOOLAEGHE E, RANJAN R, BOSMANS E, BERGMANS R, DESNYDER R. Increased serum interleukin-1-receptor-antagonist concentrations in major depression[J]. Journal of Affective Disorders, 1995, 36(1/2): 29-36. |
| [16] |
LASSELIN J, LEKANDER M, BENSON S, SCHEDLOWSKI M, ENGLER H. Sick for science: experimental endotoxemia as a translational tool to develop and test new therapies for inflammation-associated depression[J]. Molecular Psychiatry, 2021, 26(8): 3672-3683. DOI:10.1038/s41380-020-00869-2 |
| [17] |
DANTZER R, O'CONNOR JC, FREUND GG, JOHNSON RW, KELLEY KW. From inflammation to sickness and depression: when the immune system subjugates the brain[J]. Nature Reviews Neuroscience, 2008, 9(1): 46-56. DOI:10.1038/nrn2297 |
| [18] |
KÖHLER CA, FREITAS TH, MAES M, DE ANDRADE NQ, LIU CS, FERNANDES BS, STUBBS B, SOLMI M, VERONESE N, HERRMANN N, RAISON CL, MILLER BJ, LANCTÔT KL, CARVALHO AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies[J]. Acta Psychiatrica Scandinavica, 2017, 135(5): 373-387. DOI:10.1111/acps.12698 |
| [19] |
ENACHE D, PARIANTE CM, MONDELLI V. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue[J]. Brain, Behavior, and Immunity, 2019, 81: 24-40. |
| [20] |
PEARSON TA, MENSAH GA, ANDERSON JL, CANNON RO 3rd, CRIQUI M, FADL YY, FORTMANN SP, HONG YL, MYERS GL, RIFAI N, SMITH SC Jr, TAUBERT K, TRACY RP, VINICOR F, Centers for Disease Control and Prevention, ASSOCIATION AH. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association[J]. Circulation, 2003, 107(3): 499-511. DOI:10.1161/01.CIR.0000052939.59093.45 |
| [21] |
YUAN XX, WANG YP, LI X, JIANG JJ, KANG YL, PANG LJ, ZHANG PF, LI A, LV LX, ANDREASSEN OA, FAN XD, HU SH, SONG XQ. Gut microbial biomarkers for the treatment response in first-episode, drug-naïve schizophrenia: a 24-week follow-up study[J]. Translational Psychiatry, 2021, 11: 422. DOI:10.1038/s41398-021-01531-3 |
| [22] |
HUANG YC, SHI X, LI ZY, SHEN Y, SHI XX, WANG LY, LI GF, YUAN Y, WANG JX, ZHANG YC, ZHAO L, ZHANG M, KANG Y, LIANG Y. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder[J]. Neuropsychiatric Disease and Treatment, 2018, 14: 3329-3337. DOI:10.2147/NDT.S188340 |
| [23] |
LIU RT, ROWAN-NASH AD, SHEEHAN AE, WALSH RFL, SANZARI CM, KORRY BJ, BELENKY P. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults[J]. Brain, Behavior, and Immunity, 2020, 88: 308-324. DOI:10.1016/j.bbi.2020.03.026 |
| [24] |
JIANG HY, LING ZX, ZHANG YH, MAO HJ, MA ZP, YIN Y, WANG WH, TANG WX, TAN ZL, SHI JF, LI LJ, RUAN B. Altered fecal microbiota composition in patients with major depressive disorder[J]. Brain, Behavior, and Immunity, 2015, 48: 186-194. DOI:10.1016/j.bbi.2015.03.016 |
| [25] |
KNUDSEN JK, BUNDGAARD-NIELSEN C, HJERRILD S, NIELSEN RE, LEUTSCHER P, SØRENSEN S. Gut microbiota variations in patients diagnosed with major depressive disorder-a systematic review[J]. Brain and Behavior, 2021, 11(7): e02177. |
| [26] |
YATSUNENKO T, REY FE, MANARY MJ, TREHAN I, DOMINGUEZ-BELLO MG, CONTRERAS M, MAGRIS M, HIDALGO G, BALDASSANO RN, ANOKHIN AP, HEATH AC, WARNER B, REEDER J, KUCZYNSKI J, CAPORASO JG, LOZUPONE CA, LAUBER C, CLEMENTE JC, KNIGHTS D, KNIGHT R, et al. Human gut microbiome viewed across age and geography[J]. Nature, 2012, 486(7402): 222-227. DOI:10.1038/nature11053 |
| [27] |
KOVTUN AS, AVERINA OV, ANGELOVA IY, YUNES RA, ZORKINA YA, MOROZOVA AY, PAVLICHENKO AV, SYUNYAKOV TS, KARPENKO OA, KOSTYUK GP, DANILENKO VN. Alterations of the composition and neurometabolic profile of human gut microbiota in major depressive disorder[J]. Biomedicines, 2022, 10(9): 2162. DOI:10.3390/biomedicines10092162 |
| [28] |
CHEUNG SG, GOLDENTHAL AR, UHLEMANN AC, MANN JJ, MILLER JM, SUBLETTE ME. Systematic review of gut microbiota and major depression[J]. Frontiers in Psychiatry, 2019, 10: 34. DOI:10.3389/fpsyt.2019.00034 |
| [29] |
SANADA K, NAKAJIMA S, KUROKAWA S, BARCELÓ-SOLER A, IKUSE D, HIRATA A, YOSHIZAWA A, TOMIZAWA Y, SALAS-VALERO M, NODA Y, MIMURA M, IWANAMI A, KISHIMOTO T. Gut microbiota and major depressive disorder: a systematic review and meta-analysis[J]. Journal of Affective Disorders, 2020, 266: 1-13. DOI:10.1016/j.jad.2020.01.102 |
| [30] |
RONG H, XU D, LIU TB, WANG MB, ZHAO J, LIU YH, YANG HC, ZHANG J. A case-control study on the abundance, species and gene functional pathway of gut microbiota in patients with depression[J]. Chinese Journal of Psychiatry, 2017, 50(3): 208-213. (in Chinese) 荣晗, 徐丹, 刘铁榜, 王明帮, 赵杰, 刘泱慧, 杨海晨, 张建. 抑郁症患者肠道菌群丰度和种类及基因功能通路的病例对照研究[J]. 中华精神科杂志, 2017, 50(3): 208-213. DOI:10.3760/cma.j.issn.1006-7884.2017.03.010 |
| [31] |
HUANG TT, LAI JB, DU YL, XU Y, RUAN LM, HU SH. Current understanding of gut microbiota in mood disorders: an update of human studies[J]. Frontiers in Genetics, 2019, 10: 98. DOI:10.3389/fgene.2019.00098 |
| [32] |
SHEN ZH, ZHU CX, QUAN YS, YANG JM, YUAN W, YANG ZY, WU S, LUO WW, TAN B, WANG XY. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses[J]. Journal of Gastroenterology and Hepatology, 2018, 33(10): 1751-1760. DOI:10.1111/jgh.14144 |
| [33] |
TAMANAI-SHACOORI Z, SMIDA I, BOUSARGHIN L, LOREAL O, MEURIC V, BING FONG S, BONNAURE-MALLET M, JOLIVET-GOUGEON A. Roseburia spp. : a marker of health?[J]. Future Microbiology, 2017, 12(2): 157-170. DOI:10.2217/fmb-2016-0130 |
| [34] |
CANFORA EE, JOCKEN JW, BLAAK EE. Short-chain fatty acids in control of body weight and insulin sensitivity[J]. Nature Reviews Endocrinology, 2015, 11(10): 577-591. DOI:10.1038/nrendo.2015.128 |
| [35] |
HIIPPALA K, KAINULAINEN V, KALLIOMÄKI M, ARKKILA P, SATOKARI R. Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp.[J]. Frontiers in Microbiology, 2016, 7: 1706. |
| [36] |
MORGAN XC, KABAKCHIEV B, WALDRON L, TYLER AD, TICKLE TL, MILGROM R, STEMPAK JM, GEVERS D, XAVIER RJ, SILVERBERG MS, HUTTENHOWER C. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease[J]. Genome Biology, 2015, 16(1): 67. DOI:10.1186/s13059-015-0637-x |
| [37] |
BUTERA A, DI PAOLA M, VITALI F, DE NITTO D, COVOTTA F, BORRINI F, PICA R, DE FILIPPO C, CAVALIERI D, GIULIANI A, PRONIO A, BOIRIVANT M. IL-13 mRNA tissue content identifies two subsets of adult ulcerative colitis patients with different clinical and mucosa-associated microbiota profiles[J]. Journal of Crohn's and Colitis, 2020, 14(3): 369-380. DOI:10.1093/ecco-jcc/jjz154 |
| [38] |
SAKAMOTO M, BENNO Y. Reclassification of Bacteroides distasonis, Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen. nov., comb. nov., Parabacteroides goldsteinii comb. nov. and Parabacteroides merdae comb. nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2006, 56(7): 1599-1605. |
| [39] |
KVERKA M, ZAKOSTELSKA Z, KLIMESOVA K, SOKOL D, HUDCOVIC T, HRNCIR T, ROSSMANN P, MRAZEK J, KOPECNY J, VERDU EF, TLASKALOVA-HOGENOVA H. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition[J]. Clinical and Experimental Immunology, 2011, 163(2): 250-259. |
| [40] |
FU XD, LIU ZM, ZHU CL, MOU HJ, KONG Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria[J]. Critical Reviews in Food Science and Nutrition, 2019, 59(sup1): S130-S152. |
 2023, Vol. 50
2023, Vol. 50