扩展功能
文章信息
- 杨晓莉, 曹兴圆, 吴博贤, 徐其静, 刘雪
- YANG Xiaoli, CAO Xingyuan, WU Boxian, XU Qijing, LIU Xue
- 土壤中微生物植酸酶的活性及其提高方法与应用
- Microbial phytase activity in soils and methods to improve the activity and application: a review
- 微生物学通报, 2023, 50(6): 2687-2708
- Microbiology China, 2023, 50(6): 2687-2708
- DOI: 10.13344/j.microbiol.china.220763
-
文章历史
- 收稿日期: 2022-08-15
- 接受日期: 2022-09-16
- 网络首发日期: 2022-11-26
2. 西南林业大学环境修复与健康研究院,云南 昆明 650224
2. Institute of Environment Remediation and Health, Southwest Forestry University, Kunming 650224, Yunnan, China
磷(P)是植物生长发育必需的大量营养元素,影响植物生理生态,也是农业生产的限制性元素[1]。植物主要从土壤内源磷库和外源磷肥中获得磷。磷肥的主要生产原料磷矿石是有限不可再生资源[2]。磷肥进入土壤后极易被土壤强烈吸附,形成难以被植物利用的形态,在土壤中稳定存在,仅约1%可被植物直接吸收利用[3]。由于磷肥利用率低,为保障农作物营养和产量,通常施用过量磷肥,长期施肥导致磷在土壤中大量积累,农田系统中磷流失可引起潜在水体污染风险[4]。
土壤有机磷是土壤磷的重要组成部分,土壤有机磷占总磷的20%−80%[5],是植物生长所需磷的重要供给源[6]。土壤有机磷主要为植酸(phytate)、磷脂和核酸等,其中植酸磷占比50%−80%[7]。植酸磷不能被植物直接吸收利用[8],但研究表明,以植酸磷作唯一磷源时植物能正常生长,说明植酸磷是植物的潜在磷源[9]。土壤中植酸主要来源于动植物残体分解和无机磷转化。通常,植酸主要存在于谷物和种子中,是种子萌发所需磷和肌醇的储备库,种子中植酸磷占总磷的80%[10]。每分子植酸含有6个磷酸基团和12个可解离质子,易螯合二价金属阳离子Ca2+、Mg2+、Fe2+、Zn2+、Cu2+等,形成难溶性或不溶性植酸盐络合物[11],降低植酸和矿质元素的生物可利用性[12],因此植酸是一种抗营养物质。土壤中植酸盐需在专一性酶植酸酶(phytase)的作用下,通过脱磷酸化水解反应释放无机磷[13],但植酸酶酶活易受环境因子影响,导致水解效率低和土壤可利用态磷缺乏[14]。
植酸酶是一种磷酸单酯水解酶,是催化植酸及其盐类水解为肌醇与磷酸(盐)的一类酶的总称。植酸酶含量和活性被认为是影响土壤植酸盐水解和有效态磷含量的关键因素[10]。此外,植酸盐水解也可释放螯合的金属矿质元素,可同时为植物提供磷素和矿质元素,促进植物生长[15-16]。已发现微生物和植物根系均可分泌植酸酶,然而,土壤微生物和植物植酸酶含量与植物生长发育无显著相关性[17],表明植酸酶进入土壤后其活性受土壤环境因子的显著影响。此外,植酸酶的水解效率也受水解底物——植酸盐的种类和有效性的影响。土壤植酸酶主要来源于微生物,产植酸酶的微生物主要包括真菌、细菌和酵母菌[18]。微生物植酸酶虽已进行工业化生产,但由于植酸酶种类及酶学性质等差异性造成其实际应用的局限性,主要表现为热稳定性差和活性低。如何提高植酸酶在土壤中的催化活性已引起关注,主要包括获得热稳定性植酸酶、提高酶在土壤中的稳定性和酶活性。本文基于前期研究,综述微生物植酸酶的来源与分类、水解作用机制及土壤中植酸酶活性的影响因素(温度、pH、土壤吸附、钙含量及钙磷比、底物浓度、含量和有效性等),重点阐述保持或提高土壤中植酸酶活性的方法技术(纳米材料负载植酸酶、提高植酸酶pH适应范围、定点突变等)及实际应用效率,以期为微生物植酸酶应用于农业生产和提高土壤难利用态植酸磷的利用效率提供理论基础和技术参考,对降低外源磷肥施用和环境磷污染具有重要的现实意义。
1 微生物植酸酶的来源与分类 1.1 来源植酸酶广泛分布于自然界,在动物、植物和微生物中均已发现,动物植酸酶含量低,植物植酸酶易受加工、贮存过程影响且难以提纯,而微生物植酸酶由于生长周期短、pH和温度适应范围广(2.0−8.5, 20−80 ℃)、活性高(811−1 800 U/mg)、易于生产、产量高且分离提纯较容易等,是植酸酶最易得和最经济的来源[19-20]。土壤中植酸酶主要由微生物分泌产生,主要包括真菌中的曲霉属(Aspergillus),如黑曲霉(Aspergillus niger)、烟曲霉(Aspergillus fumigatus)、米曲霉(Aspergillus orgaze)、土曲霉(Aspergillus terms)、无花果曲霉(Aspergillus ficuum)等,以及青霉菌属(Penicillium)和毛霉菌属(Mucor)均可分泌高活性的胞外植酸酶[21-22]。其中,黑曲霉(A. niger)和烟曲霉(A. fumigatus)具有较强的植酸酶生产能力,被认为是植酸酶的主要生产菌[23]。例如,黑曲霉(A. niger)植酸酶活性可达50−103 U/mg,具有较宽的pH适宜范围(2.5−7.5,最适pH为4.6),较好的热稳定性(25−70 ℃,最适温度为60 ℃)[24-25]。烟曲霉(A. fumigatus)植酸酶活性为23−28 U/mg[25],具有较宽的pH适宜范围(2.5−8.0)、较好的热稳定性(50−90 ℃,最适温度70 ℃)和较广泛的底物特异性(与一系列在结构上与植酸相似的磷酸盐化合物,如苯基磷酸盐、对硝基苯基磷酸盐和磷酸烯醇丙酮酸均具有活性、易水解植酸为肌醇2-单磷酸,中间产物无大量积累和易水解肌醇1-单磷酸)[26]。细菌植酸酶通常在胞内,但肠杆菌属(Enterobacter)、芽孢杆菌属(Bacillus)和克雷伯氏菌属(Klebsiella)可产生胞外植酸酶[25, 27-28],如大肠杆菌(Escherichia coli)、枯草芽孢杆菌(Bacillus subtilis)和土生克雷伯氏菌(Klebsiella terrigena) (表 1)。其中大肠杆菌(E. coli)胞外植酸酶活性可达811−1 800 U/mg、最适pH值为4.5,最适温度65 ℃[27]。枯草芽孢杆菌(B. subtilis)植酸酶活性为9−15 U/mg,最适pH为7.0,最适温度为55 ℃[29, 51]。微生物植酸酶由于具有较高的水解活性且易得,已通过生物技术进行广泛商业化生产[18]。
| 微生物植酸酶种类 Microbial phytase species |
微生物植酸酶来源 Microbial phytase sources |
最适pH Optimum pH |
最适温度 Optimum temperature (℃) |
结构特征 Structure characteristics |
催化位置 Catalytic sites |
| 3-植酸酶(EC 3.1.3.8) 3-phytase |
土生克雷伯氏菌[28] K. terrigena[28] | 5.0 | 58 | 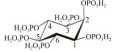 由肌醇环和6个磷酸基团通过磷酸单酯键连接构成[31] Consisting of an inositol ring and 6 phosphate groups bound by a phosphate monoester bond[31] |
植酸酶的植酸降解途径,肌醇依次从C3、C4、C5、C6、C1位游离磷酸基团生成I(2)P[32] In phytic acid hydrolysis pathway by phytase, inositol generates I(2)P from free phosphate groups at C3, C4, C5, C6 and C1 sites[32] |
| 黑曲霉[24] A. niger[24] | 4.6 | 60 | |||
| 烟曲霉[29] A. fumigatus[29] | 7.5 | 40 | |||
| 卡氏德巴利酵母[30] Debaryomyces castellii CBS 2923[30] | − | − | |||
| 5-植酸酶(EC 3.1.3.72) 5-phytase |
反刍兽新月形单胞菌属[33] Crotonomonas ruminants[33] |
5.0−5.5 | 45 | 植酸酶依次从植酸C5、C4、C6、C3、C1位释放磷酸基团,终产物为I(2)P[33] Phytase releases phosphate groups from C5, C4, C6, C3 and C1 of phytic acid in order. The final product is I(2)P[33] |
|
| 6-植酸酶(EC 3.1.3.26) 6-phytase |
隔孢伏革菌[34] Peniophora lycii[34] | 5.0−5.5 | 45 | 肌醇从植酸C6、C5、C4、C6、C3、C1位释放磷酸基团,终产物为I(2)P[35-36] Inositol releases phosphate groups from C6, C5, C4, C6, C3 and C1 of phytic acid and the final product is I(2)P[35-36] |
|
| 组氨酸酸性磷酸酶植酸酶 | 无丙二酸柠檬酸杆菌[37] Citrobacter amalonaticus CGMCC 1696[37] | 4.5 | 55 | 具有保守催化基元RHGXRXP和HD[32] | 氨基酸序列由N-末端基元RHGXRXP和C-末端基元HD组成,正确折叠时这两个远距离基元会聚形成1个特异的催化中心,起动两步反应水解磷酸单酯[40] |
| Histidine acid phosphatase phytase (HAP) | 奥默柯达酵母菌[38] Kodamaea ohmeri BG3[38] | 5.5 | 65 | Conserved catalytic units RHGXRXP and HD[32] | The amino acid sequence consists of an N-terminal unit RHGXRXP and a C-terminal unit HD. When correctly folded, these two remote units converge to form a specific catalytic center and initiate a two-step hydrolysis of phosphate monoester[40] |
| 微细正青霉[39] Eupenicillium parvum BCC 17694[39] | 5.5 | 50 | |||
| 大肠杆菌[27] Escherichia coli[27] |
4.5 | 65 | |||
| β-螺旋桨植酸酶 β-propeller phytase (BPP) |
枯草芽孢杆菌[29] Bacillus subtilis[29] | 7.0 | 55 | 由β-折叠片组成,类似六叶螺旋桨[42] Composed of β-sheets, similar to a six-bladed propeller[42] |
BPP具有2个不对称磷酸基团结合部位,“切割部位” (cleavage site, CS)和“亲和部位” (affinity site, AS)。AS促进植酸分子结合,而CS负责水解磷酸基团[43] BPP has two asymmetric phosphate binding sites, ‘Cleavage site’ (CS) and ‘Affinity site’ (AS). AS promotes binding of phytic acid molecules while CS is responsible for hydrolysis of phosphate groups[43] |
| 解淀粉芽孢杆菌[41] Bacillus amyloliquefaciens[41] |
7.0−8.0 | 70 | |||
| 半胱氨酸磷酸酶植酸酶 Cysteine phosphatase phytase (CP) |
反刍兽新月形单胞菌[44] Selenomonas ruminantium SrPf6[44] | 2.6−6.0 | 50−55 | 氨基酸序列包含活性部位基元HCXXGXXR (T/S),并与CP类的蛋白酪氨酸磷酸具有相似性[45] The amino acid sequence contains the active site motif HCXXGXXR (T/S) and is similar to CP tyrosine phosphates[45] |
形成一个含保守半胱氨基酸(C241)的磷酸基团结合环(phosphate-bindingloop, P-loop),其中C241不可逆氧化使P-loop由非活性的开放构象转变为活性的闭合构象[46] Forms a phosphate-binding loop (P-loop), containing a conserved cysteic amino acid (C241), in which irreversible oxidation of C241 converts the P-loop from an inactive open conformation to an active closed conformation[46] |
| 紫色酸性磷酸酶植酸酶 Purple acid phosphatase phytase (PAP) |
烟草(Nicotiana tabacum)根系分泌PAPhy[47] N. tabacum root-exudated PAPhy[47] |
5.0−5.5 | 45 | 7个残基(D、D、Y、N、H、H、H)包含在5个保守基元(DXG/GDXXY/ GNH(ED)/VXXH/ GHXH)中[50] 7 residues (D, D, Y, N, H, H, H) contained in 5 conserved units (DXG/GDXXY/GNH (ED)/VXXH/GHXH)[50] |
保守基元(DXG/GDXXY/GNH(ED)/ VXXH/GHXH)[50] Conserved units (DXG/GDXXY/GNH(ED)/ VXXH/GHXH)[50] |
| 拟南芥[48] Arabidopsis thaliana[48] |
4.5 | 25−37 | |||
| 新洋葱伯克霍尔德菌[49] Burkholderia cenocepacia[49] |
− | − | |||
|
−:文献中无确切信息 −: There is no information in the citations. |
|||||
植酸酶种类可根据不同标准进行分类,根据最适pH可分为酸性植酸酶(acidic phytase)和碱性植酸酶(alkaline phytase);根据植酸水解位点特异性可分为3-植酸酶(EC 3.1.3.8)、6-植酸酶(EC 3.1.3.26)和5-植酸酶(EC 3.1.3.72)[52];根据蛋白结构和催化机理可分为组氨酸植酸酶(histidine acid phosphatase phytase, HAP)、β-螺旋桨植酸酶(β-propeller phytase, BPP)、半胱氨酸植酸酶(cysteine phosphatase phytase, CP)和紫色酸性磷酸酶植酸酶(purple acid phosphatase phytase, PAP)[53] (表 1)。
2 植酸酶的作用机理植酸酶可专一性水解植酸分子中的磷酯键,水解过程分步进行,逐个释放磷酸基团,始于完全磷酸化的六磷酸肌醇(IP6),过程中形成肌醇五磷酸酯至肌醇一磷酸酯中间产物(IP5−IP1),终产物为肌醇和磷酸根[54]。
不同来源植酸酶的水解作用机理不同,起始水解酯键不同。微生物3-植酸酶首先从植酸的第3碳位点开始水解酯键释放无机磷,继而依次释放其他碳位点磷,最终酯解植酸分子,作用过程为:植酸→d/l-1, 2, 4, 5, 6-五磷酸肌醇→ d/l-1, 2, 5, 6-四磷酸肌醇→d/l-1, 2, 5-三磷酸肌醇、d/l-1, 2, 6-三磷酸肌醇、d/l-2, 5, 6-三磷酸肌醇→d/l-2, 5-二磷酸肌醇、d/l-1, 2-二磷酸肌醇、d/l-2, 6-二磷酸肌醇→2-磷酸肌醇[35, 55] (图 1)。

|
| 图 1 3-植酸酶对植酸的脱磷酸化过程 Figure 1 Dephosphorylation of phytate by 3-phytase. |
|
|
来源少数细菌的5-植酸酶可优先从植酸的第5碳位点开始水解酯键释放无机磷,其作用过程为:植酸→1, 2, 3, 4, 6-五磷酸肌醇→ d/l-1, 2, 3, 4-四磷酸肌醇、d/l-1, 2, 3, 6-四磷酸肌醇→d/l-1, 2, 3-三磷酸肌醇→d/l-1, 2-二磷酸肌醇→2-磷酸肌醇[55] (图 2)。
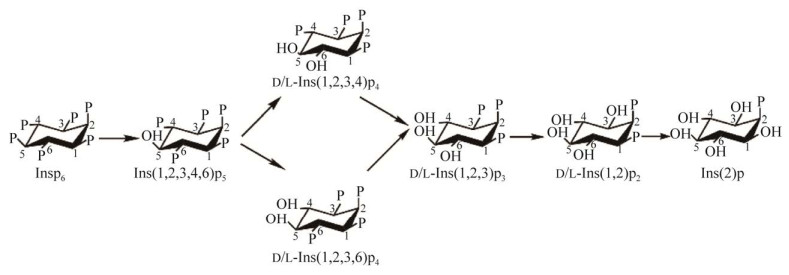
|
| 图 2 5-植酸酶对植酸的脱磷酸化过程 Figure 2 Dephosphorylation of phytate by 5-phytase. |
|
|
6-植酸酶主要来源于植物,在担子菌中也有报道[32, 56]。6-植酸酶首先在植酸的第6碳位点开始水解催化而释放无机磷,其作用过程为:植酸→l-1, 2, 3, 4, 5-五磷酸肌醇、d-1, 2, 4, 5, 6-五磷酸肌醇→l-l, 2, 3, 4-四磷酸肌醇、d-1, 2, 5, 6-四磷酸肌醇→l-1, 2, 3-三磷酸肌醇、d-1, 2, 6-三磷酸肌醇→d/l-1, 2-二磷酸肌醇→d/l-1-磷酸肌醇、d/l-2-磷酸肌醇→肌醇[55] (图 3)。

|
| 图 3 6-植酸酶对植酸的脱磷酸化过程 Figure 3 Dephosphorylation of phytate by 6-phytase. |
|
|
植酸酶活性以一定pH值(5.5)和温度(37 ℃)条件下每分钟从植酸底物中释放无机磷的含量来表征,表示为U。土壤微生物植酸酶活性因分子结构不同而产生差异[57],且不同来源植酸酶活性的影响因素也存在较大差异。微生物植酸酶活性与植物磷营养无显著相关性,表明植酸酶进入土壤后其活性降低,主要影响因素为温度、pH值、土壤吸附、钙含量及钙磷比、底物种类、含量和有效性等[58]。
3.1 温度温度是影响植酸酶活性的关键因素[59]。植酸酶一般在25−70 ℃范围内具有活性[60],最适温度一般为44−60 ℃[61]。不同来源植酸酶的活性对温度适应性差异较大。植物性植酸酶最适温度为45−60 ℃[62],微生物植酸酶一般为45−55 ℃[63],其中土生克雷伯氏菌(K. terrigena)植酸酶最适温度为37 ℃和55 ℃[64],但土壤放线菌(Actinomycete)胞外植酸酶最适温度为70 ℃[65]。在一定温度范围内,酶活性随温度升高而提高,但当温度升高到一定值(80 ℃)时,酶活性降低至失活。例如,无花果曲霉(A. ficunm)植酸酶于80 ℃加热3 min后酶活降低了82%,加热5 min后其活性降为0[66]。尹德胜等[67]研究表明,pH 7.8条件下,无花果曲霉(A. ficunm)植酸酶最适温度为50 ℃,50−55 ℃范围内活性较高(> 90%),65 ℃时酶活性降至70%,70 ℃时降至55%。Wyss等[68]研究表明,75 ℃时烟曲霉(A. fumigatus)和黑曲霉(A. niger)的植酸酶活性降至63%−73%,85 ℃时降至31%−51%。也有报道指出,黑曲霉(A. niger) 9701植酸酶具有较好的热稳定性,90 ℃加热15 min后仍保留75%的酶活性,30 min后酶活仍可保持84%[28]。烟曲霉(A. fumigatus)植酸酶基因在黑曲霉(A. niger) NW205中表达后,获得的重组酶在100 ℃加热20 min后酶活仅损失10%,具有极好的热稳定性[69]。
3.2 pH值植酸酶pH值活性范围一般为2.0−8.5,通常植酸酶活性最佳的pH值范围为4.5−6.0[70]。不同来源植酸酶的最适pH值差异较大。植物性植酸酶活性pH值范围为4.0−6.0[71],最适pH值为5.5,< 4.0或 > 7.0植酸酶基本失活[62],而豆科植物种子、百合花粉、香蒲植酸酶在pH 8.0时仍具有活性[17]。微生物植酸酶pH活性范围较宽,黑曲霉(A. niger)植酸酶具有较宽的pH适宜范围(2.5−7.5,最适pH值为4.6)[24]。无花果曲霉(A. ficunm)植酸酶最适pH值为7.8,pH 9.0时酶活降至7%[67]。Greiner[72]研究表明,成团泛菌(Pantoea agglomerans)植酸酶在pH 2.5−7.5和温度4 ℃条件下较稳定,最适pH值为4.5,10 d时酶活性仍可保留95%以上(pH 4.5),但pH < 2.0或pH > 8.0时酶活性迅速下降,如pH 1.5时剩余酶活为85%,pH 9.0时酶活在24 h内降低了70%。
3.3 土壤吸附土壤中植酸酶的稳定性和活性受土壤颗粒/有机矿物表面吸附的影响[73],植酸酶的吸附可降低其对底物的亲和力,从而降低其有效活性。例如,将植酸酶添加到土壤中后,其活性在8 min内迅速下降至初始活性的25%以下,并且活性随时间的延长持续下降[74]。黑曲霉(A. niger)游离植酸酶活性为80% (pH 4.5−5.5),土壤吸附后酶活降至37.3%[75]。将黑曲霉(A. niger)植酸酶与针铁矿和赤铁矿混合,植酸酶活性随时间延长显著降低,吸附24 h后酶活降低了50%[76],吸附后植酸酶发生构象变化从而导致部分或全部发生不可逆失活。通常情况下,土壤对植酸酶的吸附在pH 4.5时最高,随着pH升高,吸附逐渐减少,pH 7.5时植酸酶完全存在于土壤溶液中[77]。植酸酶稳定性和活性受土壤类型和土壤矿物组成的影响。例如,吸附28 d后,沙质土壤中植酸酶活性可保留40%,而黏土中活性仅为5%[77]。此外,被黏土矿物吸附后,植酸酶活性最适pH值升高[78],因最适pH值变化进而影响植酸酶在土壤中的稳定性和活性,导致对植酸盐的水解效率发生变化[79]。
3.4 钙含量及钙磷比Ca2+维持细胞结构稳定性和细胞内离子平衡,作为重要的第二信使偶联胞外信号与胞内生理反应,是植物代谢和生长发育的主要调控因子[80]。植酸酶基因呈Ca2+端依赖催化特性,但高Ca2+ (5 mmol/L)抑制植酸酶的活性,10 mmol/L Ca2+导致植酸酶完全失活,产生这一现象的原因为过量Ca2+占据酶的活性位点,阻碍植酸酶与植酸底物的接触和反应[81]。研究表明,植酸酶活性受Ca2+质量浓度的影响,当Ca2+质量浓度为1%−2%时酶活较低(445−450 U/mL),Ca2+质量浓度为3%时酶活最高(480 U/mL),Ca2+质量浓度为4%时酶活降为420 U/mL[82]。类似地,当Ca2+质量浓度增加至0.1%、0.4%和0.9%时,植酸酶活性分别降低了52%、67%和85%[83],表明随Ca2+质量浓度升高,Ca2+对植酸酶产生竞争性抑制,因此高质量浓度Ca2+不利于植酸酶活性发挥。钙磷摩尔比为1.1:1−1.4:1时植酸酶活性较高,提高钙磷摩尔比导致植酸酶活性降低[84]。例如,钙磷摩尔比由1.4:1提高至2:1时,植酸酶活性降低了7.4%[85];钙磷摩尔比由2:1降至1.1:1时,植酸酶活性提高了5%−12%[86];钙磷摩尔比由2.1:1降至1.2:l时,植酸酶活性提高了16%[87]。产生该现象的可能原因是:(1) 钙与植酸可形成植酸钙沉淀或钙-植酸络合物[88],导致植酸可利用性和有效性降低,影响植酸酶对植酸的水解效率[87],从而导致植酸酶活性降低;(2) 高钙竞争植酸酶的活性位点,直接降低植酸酶的活性和水解效率。
3.5 底物种类、含量和有效性植酸酶活性受水解植酸盐底物种类、含量和有效性的影响。Greiner[72]通过对14种底物的水解效率(37 ℃,pH 4.5)分析发现,成团泛菌(P. agglomerans)植酸酶对不同底物的活性存在差异,其中对植酸、葡萄糖-1-磷酸酯、氢化钠-焦磷酸盐和1-磷酸萘酯表现出较高活性,米氏常数Km分别为0.34、0.26、0.98和1.10 mmol/L,酶转化数kcat分别为21、101、15和6.1 s−1,总催化效率kcat/Km分别为61 765、384 615、15 406和5 596 L/(mmol·s)。此外,植酸酶活性受底物浓度的影响,酶促反应速率与底物浓度密切相关,当酶浓度、温度和pH恒定、底物浓度较低(0−4.8 mmol/L)时,酶促反应速率与底物浓度呈正相关,底物浓度提高至4.8−6.6 mmol/L时反应速率基本无变化,维持平稳状态;随底物浓度继续升高,酶促反应速率增幅下降,底物浓度达到一定临界浓度(6.6 mmol/L)时反应速率下降,原因是植酸酶被底物浓度饱和,达到反应平衡,表明高底物浓度对植酸酶的催化活性具有不利影响[89]。
土壤中植酸盐的活动性和有效性也对植酸酶的水解效率具有一定影响,土壤中植酸盐的有效性受有机质、黏土类型、pH和金属氧化物等因素的影响[90-91]。其中,黏土类型影响植酸的吸附强度,如伊利石、高岭石和蒙脱石、针铁矿[92]、赤铁矿[93]、氢氧化铝对植酸具有很强的吸附作用[94],植酸在针铁矿上的吸附量显著高于无机磷(3.8−12.7 vs. 2.4−4.6 µmol/m2),其与无结晶铝氧化物的结合诱导形成稳定的植酸铝沉淀(log10 K13‒16=8.84−20.1)[95-96]。植酸与重金属离子的络合作用表现为Al3+ > Fe3+ > Mg2+ > Fe2+ > Ca2+[97],其稳定常数log10 K13‒15分别为12.2−20.1、8.89−18.2、8.76−10.5、7.71−10.5和8.3−8.4[98]。此外,随pH值升高,植酸吸附量则降低。例如,pH 4.5时植酸在铁氢化物表面的吸附量为2.12 µmol/m2[99],pH值升高至6.5时吸附量降低了50%[96],这是因为植酸与Fe、Al、Ca和Mg的吸附/络合反应受pH影响,酸性(pH 5.0)条件下易与Fe/Al形成沉淀,碱性条件(pH 7.5)下易与Ca/Mg形成沉淀[79, 100-101]。
4 提高植酸酶活性的方法及其应用 4.1 纳米材料负载植酸酶纳米材料负载植酸酶可降低土壤颗粒对植酸酶的吸附,提高植酸酶在土壤溶液中的稳定性,使其发挥活性。近几年的研究表明,羟基磷灰石(hydroxyapatite, HA)作为一种无机固体材料,具有良好的生物相容性、强亲和力和无毒等特点,可制成纳米颗粒,通过快速吸附将植酸酶负载固定,固定率近100%且负载后酶活未损失[102]。HA中Ca2+与酶的羧酸基团螯合反应迅速,产生高度稳定的固定化酶,对植酸酶在土壤中发挥活性具有一定应用前景。与游离植酸酶相比,固定化/负载植酸酶在一定pH和温度范围内可保持较高活性,在高温下可保持较高的稳定性。例如,相较于游离植酸酶,负载酶在pH 3.0时活性由40%提高至90%,pH 7.0时活性由23%提高至38%;80−90 ℃时,游离植酸酶发生变性,20 min内完全失活,而负载植酸酶在80 ℃、3 h和90 ℃、20 min时,活性仍可保持60%和40%[103]。纳米材料负载植酸酶目前主要应用于饲料添加剂以提高饲料中植酸磷的利用率,减少家禽(特别是单胃动物)未消化植酸磷向环境中的排放[104] (表 2)。后续可考虑将纳米材料负载植酸酶应用于土壤,提高植物/作物对土壤植酸磷的利用率,以降低外源磷肥施加,同时降低土壤和水环境的磷污染风险。
| 方法 Methods |
材料 Materials |
技术 Technology |
酶活特性 Characteristics of enzyme activity |
应用 Application |
参考文献 References |
| 纳米材料负载植酸酶 Nanomaterial loaded phytase |
羟基磷灰石 Hydroxyapatite (HA) |
吸附、固定 Adsorption and fixation |
负载酶在pH 3.0时活性由40%提高至90%,pH 7.0时活性由23%提高至38%;80−90 ℃时,游离植酸酶发生变性,20 min内完全失活,而负载酶在80 ℃、3 h和90 ℃、20 min时,活性仍可保持60%和40% The activity of loaded enzyme is increased from 40% to 90% at pH 3.0 and from 23% to 38% at pH 7.0; At 80−90 ℃, the free phytase is denatured and completely inactivated within 20 min, while the activity of the loaded enzyme remained 60% and 40% at 80 ℃, 3 h and 90 ℃, 20 min |
土壤改良剂 Soil amendment |
[103-104] |
| 氧化石墨烯 Graphene oxide (GO) |
负载植酸酶活性在42 ℃和85 ℃分别提高40倍和20倍 The activity of loaded phytase is increased by 40 and 20 times at 42 ℃ and 85 ℃, respectively |
开发耐热植酸酶 Develop heat- resistant phytase |
[105] | ||
| 壳聚糖 Chitosan |
包埋,壳聚糖纳米粒将植酸酶包封 Embedding, chitosan nanoparticles encapsulate phytase |
负载酶适宜温度变广,70 ℃加热30 min后,游离植酸酶活性为21.4%,负载酶活性为63.3%;90 ℃时,游离植酸酶失活,负载酶活性仍可保持11.4% The optimum temperature of the loaded enzyme is wide. After heating at 70 ℃ for 30 min, the free phytase activity is reduced to 21.4% and loaded enzyme activity remains 63.3%; At 90 ℃, free phytase was inactivated and the loaded enzyme activity remains 11.4% |
生物修复和生物转化 Bioremediation and biotransformation |
[106] | |
| 负载酶的活性提高,米氏常数Km由34 μmol/L提高至56 μmol/L,最大反应速率Vmax由120 μmol/(min·mg)提高至401 μmol/(min·mg),固定化植酸酶的活性可达50.4% The activity of loaded enzyme is increased, with Km increased from 34 μmol/L to 56 μmol/L, the maximum reaction rate Vmax increases from 120 μmol/(min·mg) to 401 μmol/(min·mg), and the immobilized phytase activity reaches 50.4% |
[107] | ||||
| 植酸酶酶活可达1 665 U Phytase activity reaches 1 665 U |
[108] | ||||
| 提高pH适应范围 Improve pH adaptations |
黑曲霉A. niger | 基因重组 Genetic recombination |
重组植酸酶的最适pH范围提高至2.5−5.5和5.0−5.5,活性高达35−40 U/mg和80−90 U/mg The optimal pH range of recombinant phytase is increased to 2.5−5.5 and 5.0−5.5, and the activity reaches 35−40 U/mg and 80−90 U/mg |
促进土壤稳定态有机磷向活性有机磷和无机磷转化 Promote soil stable organic P transformation to active organic P and inorganic P |
[109] |
| 无丙二酸柠檬酸杆菌C. amalonaticus | 在酵母(P. pastoris) 表面表达 Express on yeast (P. pastoris) surface |
植酸酶活性由300 U/g提高至6 413 U/g,酶活的pH稳定性提高,pH范围由4.0−6.0提高至1.6−6.0,且酶活性由70%提高至90% Phytase activity increases from 300 U/g to 6 413 U/g, pH stability increases from 4.0−6.0 to 1.6−6.0 and the enzyme activity increases from 70% to 90% |
[19, 110] | ||
| 定点突变 Fixed point mutation |
莫氏耶尔森氏菌Yersinia mollaretii | 定点突变 Fixed point mutation |
突变株活性达993 U/mg,58 ℃加热20 min后,突变株活性是野生型的2.4倍(55% vs. 23%),强酸性条件下(pH 2.8),突变株活性是野生型的2.38倍(74% vs. 31%) The activity of the mutant reaches 993 U/mg, which is 2.4 times that of the wild type (55% vs. 23%) after heating at 58 ℃ for 20 min, and 2.38 times that of the wild type (74% vs. 31%) under strong acidic conditions (pH 2.8) |
土壤改良剂 Soil amendments |
[111] |
| 大肠杆菌E. coli AppA | 定点突变 Fixed point mutation |
突变株活性是野生型的1.5倍(175 vs. 117 U/mg),且最适温度由65 ℃提高至75 ℃,90 ℃加热15 min后植酸酶活性是野生型的3.14倍(44% vs. 14%) The activity of the mutant is 1.5 times that of the wild type (175 vs. 117 U/mg), and the optimal temperature increases from 65 ℃ to 75 ℃, mutant phytase activity is 3.14 times that of the wild type after heating at 90 ℃ for 15 min (44% vs. 14%) |
土壤改良剂 Soil amendments |
[32, 112] | |
| 随机突变 Random point mutation |
突变株I408L活性高于野生型(1 653 vs. 1 610 U/mg),85 ℃热处理5 min后活性是野生型的1.83倍(51.3% vs. 28%) The activity of mutant I408L is higher than that of wild type (1 653 vs. 1 610 U/mg), with the activity being 1.83 times that of wild type (51.3% vs. 28%) after heating at 85 ℃ for 5 min |
[113] | |||
| 大肠杆菌E. coli | 易错PCR Error prone PCR |
突变株AppA2的热稳定性提高25% (80 ℃, 10 min),催化效率较野生型[660和1 065 vs. 423 L/(μmol·s)]提高56%和152% The thermal stability of mutant AppA2 is increased by 25% (80 ℃, 10 min), and the catalytic efficiency is increased by 56% and 152% compared to wild type (660 and 1 065 vs. 423 L/(μmol·s)) |
[114] | ||
| 定点突变 Fixed point mutation |
突变株AppA2 (D144N/V227A和D144N/V227A/G344D)的热稳定性提高15% (80 ℃, 10 min),突变株催化效率是野生型的1.9倍和2.9倍[1 205和1 654 vs. 445 L/(μmol·s)],其中D144N/V227A活性(1 385 U/mg)比野生型(1 003 U/mg)提高38% The thermal stability of mutant AppA2 (D144N/V227A and D144N/V227A/ G344D) was increased by 15% (80 ℃, 10 min). The catalytic efficiency is 1.9 and 2.9 times that of the wild type (1 205 and 1 654 vs. 445 L/(μmol·s)). The activity of D144N/V227A (1 385 U/mg) is 38% higher than the wild type (1 003 U/mg) |
[115] | |||
| 定点突变 Fixed point mutation |
突变株在80 ℃加热10 min后,酶活分别为31.4%和70.5%,热稳定性比野生型提高39.1% After heating at 80 ℃ for 10 min, the enzyme activity of the mutant is 31.4% and 70.5%, respectively, and the thermal stability is 39.1% higher than the wild type |
[116] | |||
| 黑曲霉A. niger | 定点突变 Fixed point mutation |
突变株在80 ℃热处理10 min后其活性分别为466 688、438 366和503 217 U/mg The activities of the mutant are 466 688, 438 366 and 503 217 U/mg after heating at 80 ℃ for 10 min |
构建高产、高活性的超级产植酸酶菌株 Develop high yield and high phytase activity strain |
[117] | |
| 定点突变 Fixed point mutation |
突变株在80 ℃时稳定性最高,其酶活分别提高24%和22.6% The mutant shows the highest stability at 80 ℃, and its enzyme activity is increased by 24% and 22.6%, respectively |
土壤改良剂 Soil amendments |
[118] | ||
| 黑曲霉N25 A. niger N25 | 定向改造 Directional transformation |
突变株PP-NPds-12D热稳定性较高,在70、80和90 ℃水浴处理10 min,热稳定性分别提高35%、40%和31%,突变株PP-NPds-12G催化活性较高,酶活性达到112 957 U/mL,是原始菌株(513.4 U/mL)的220倍以上 The thermal stability of the mutant PP-NPds-12D is improved by 35%, 40% and 31% after heating at 70, 80 and 90 ℃ for 10 min, respectively. The catalytic activity of the mutant PP-NPds-12G reaches 112 957 U/mL, which is more than 220 times of the original strain (513.4 U/mL) |
筛选热稳定性好、催化效率高的植酸酶突变株 Screen mutants with high thermal stability and high catalytic efficiency |
[119] | |
| 黑曲霉YM-01 A. niger YM-01 | 原生质体紫外诱变 Ultraviolet mutagenesis of protoplasts |
植酸酶活性达到2 204 U/mL,突变株YM-16酶活比YM-01提高61.2%,且热稳定性提高,90 ℃高温处理10 min后,YM-16酶活为226 U/mL The phytase activity of the mutant YM-16 is 2 204 U/mL. The enzyme activity of the mutant YM-16 is 61.2% higher than YM-01, and the thermal stability is improved, which is 226 U/mL after heating at 90 ℃ for 10 min |
土壤改良剂 Soil amendments |
[120] | |
| 黑曲霉MA021 A. niger MA021 | 紫外、亚硝基胍单独和复合处理 UV/Nitrosoguanidine separate or mix-treatment |
植酸酶活性达到2 950−3 015 U/mL,是原始菌株的3.6倍 Phytase activity is 2 950−3 015 U/mL, which is 3.6 times that of the original strain |
[121] | ||
| 解淀粉芽胞杆菌DSM 1061 B. amyloliquefaciens DSM 1061 | 定点突变 Fixed point mutations |
突变株D148E活性提高35%,催化效率是野生型的1.5倍,pH 5.0−8.0范围内活性高于野生型(7.0−20.6 vs. 6.0−15 U/mg),D148E活性在65 ℃时高达20.6 U/mg。在40−75 ℃的温度范围内活性增加,突变株D148E的活性高于野生型(2.5−20.6 vs. 2−15 U/mg) The activity of mutant D148E phytase is increased by 35%, and the catalytic efficiency is 1.5 times that the wild-type, with activity is higher than wild-type (7.0−20.6 vs. 6.0−15 U/mg) at pH 5.0−8.0. The activity of D148E phytase reaches 20.6 U/mg at 65 ℃, which is higher than the wild type (2.5−20.6 vs. 2−15 U/mg) at 40−75 ℃ |
土壤改良剂 Soil amendments |
[41] | |
| 放射型根瘤杆菌A. radiobacter | 紫外-氯化锂诱变 UV-lithium chloride mutagensis |
植酸酶活性达到18.5 U/mL,比原始菌株酶活提高47.7% Phytase activity is 18.5 U/mL, which is 47.7% higher than that of the original strain |
[122-123] |
近年来,石墨烯在生物工程中的应用取得一定进展,在立体选择性和生物溶解度方面表现出一定优势,纳米技术和生物技术结合交叉表现出一定应用前景。氧化石墨烯(graphene oxide, GO)具有单层Sp2杂化结构,可用于有机生物大分子的固定化(表 2)。GO纳米载体具有较大比表面积,相较于游离植酸酶,负载植酸酶活性在42 ℃和85 ℃分别提高40倍和20倍,GO固定化技术已用于开发生产耐热植酸酶[105]。
此外,壳聚糖包埋和纳米纤维负载也可提高植酸酶在土壤中的稳定性和活性(表 2)。例如,钱浩[106]通过体外壳聚糖包埋植酸酶纳米颗粒,发现负载酶的适宜温度变广,70 ℃加热30 min后,游离植酸酶活性为21.4%,而负载酶活性为63.3%;90 ℃时,游离植酸酶失活,负载酶活性仍可保持11.4%。有研究发现,与游离植酸酶相比,纳米纤维负载酶的活性提高,米氏常数Km由34 μmol/L提高至56 μmol/L,最大反应速率Vmax由120 μmol/(min·mg)提高至401 μmol/(min·mg),但最适pH和温度无显著变化;纳米纤维固定化效率为193%,固定化植酸酶的催化活性可达50.4%,植酸酶活性的提高有助于开发用于生物修复和生物转化的技术和材料[107]。例如,王成波[108]以戊二醛为交联剂,用壳聚糖固定植酸酶,利用共价结合方法对酶的固定化进行研究,结果表明,壳聚糖负载植酸酶酶活可达1 665 U。同时,纳米材料负载植酸酶作为一种高效的固定化糖蛋白表达系统具有良好的应用前景,在畜禽和水产养殖的应用可产生较高的经济效益,且可保护生态安全,改善生态环境[124]。
4.2 提高植酸酶对土壤pH的适应范围可通过提高植酸酶对土壤pH的适应范围,以保持植酸酶在不同土壤中的活性。Bei等[109]通过重组从黑曲霉(A. niger) NRRL3135和烟曲霉(A. fumigatus) ATCC13073获得突变株植酸酶片段,获得突变株A23D5F7和AB345F7,其最适pH范围提高至2.5−5.5和5.0−5.5,活性高达35−40 U/mg和80−90 U/mg。微生物植酸酶可促进土壤稳定态有机磷向活性有机磷和无机磷转化,从而提高土壤磷的生物有效性[19] (表 2)。Li等[110]将无丙二酸柠檬酸杆菌(Citrobacter amalonaticus)植酸酶基因在毕赤酵母(P. pastoris)细胞中进行表达,使其植酸酶活性由300 U/g提高至6 413 U/g,最适温度为60 ℃,酶活pH稳定性显著提高,活性pH范围由4.0−6.0扩大至1.6−6.0,且酶活性由70%提高至90%。
4.3 定点突变定点突变是一种更快更有效提高植酸酶热稳定性和催化效率的方法。随着现代分子生物学技术的发展,采用基因工程技术手段构建植酸酶基因工程菌,可获得高产、高活性植酸酶菌株(表 2)。例如,Shivange等[111]通过突变莫氏耶尔森氏菌(Yersinia mollaretii)植酸酶,突变株活性达993 U/mg,且热稳定性和pH稳定性也提高,58 ℃加热20 min后,突变株活性是野生型的2.4倍(55% vs. 23%),强酸性条件下(pH 2.8),突变株活性是野生型的2.38倍(74% vs. 31%)。
Rodriguez等[112]通过突变大肠杆菌(E. coli) AppA植酸酶,突变株活性是野生型的1.5倍(175 U/mg vs. 117 U/mg),且最适温度由65 ℃提高至75 ℃,90 ℃加热15 min后植酸酶活性是野生型的3.14倍(44% vs. 14%)[29]。Zhu等[113]对大肠杆菌(E. coli AppA)植酸酶进行突变,突变株I408L活性高于野生型(1 653 vs. 1 610 U/mg),且热稳定性显著提高,85 ℃热处理5 min后活性是野生型的1.83倍(51.3% vs. 28%)。Kim等[114]通过易错PCR获得植酸酶AppA2突变株,与野生型相比,AppA2的热稳定性提高25% (80 ℃, 10 min),催化效率较野生型[660和1 065 vs. 423 L/(μmol·s)]提高56%和152%。类似地,Kim等[115]从大肠杆菌(E. coli)中获得了植酸酶AppA2突变株,与野生型相比,AppA2 (D144N/ V227A和D144N/V227A/G344D)的热稳定性提高15% (80 ℃, 10 min),突变株催化效率是野生型的1.9倍和2.9倍[1 205和1 654 vs. 445 L/(μmol·s)],其中D144N/V227A活性(1 385 U/mg)比野生型(1 003 U/mg)提高38%。Fei等[116]通过突变大肠杆菌(E. coli)植酸酶,在80 ℃加热10 min后,野生型AppA和突变株的酶活分别为31.4%和70.5%,突变株的热稳定性比野生型提高39.1%。
Tang等[117]通过突变黑曲霉(A. niger)植酸酶,获得突变株(Q172R、Q172R/K432R和Q368E/K432R),80 ℃热处理10 min后其植酸酶活性分别为466 688、438 366和503 217 U/mg。Hesampour等[118]通过突变黑曲霉植酸酶(A. niger PhyA),突变株P9和P12在80 ℃时稳定性最高,植酸酶酶活分别提高24%和22.6%。廖燕[119]通过突变黑曲霉(A. niger) N25植酸酶,突变株PP-NPds-12D热稳定性较高,在70、80和90 ℃水浴处理10 min,热稳定性分别提高35%、40%和31%,PP-NPds-12G催化活性较高,酶活性达到112 957 U/mL,是原始菌株(513.4 U/mL)的220倍。为进一步应用蛋白质工程技术改善植酸酶酶学性质奠定基础,筛选出热稳定性好、催化效率高的植酸酶突变株。叶明等[120]对土壤中分离的黑曲霉(A. niger) YM-01菌丝体进行原生质体紫外诱变,诱变后酶活达到2 204 U/mL,突变株YM-16酶活比YM-01提高61.2%,且热稳定性提高,90 ℃高温处理10 min后,YM-16酶活为226 U/mL,进一步对其液态产酶发酵条件进行优化,可应用于土壤酶制剂(表 2)。陈红歌等[121]对黑曲霉(A. niger) MA021进行紫外、亚硝基胍单独处理和复合处理,获得植酸酶高产菌株U-12-10,其植酸酶活性达到2 950−3 015 U/mL,是原始菌株的3.6倍。
Xu等[41]对解淀粉芽胞杆菌(B. amyloliquefaciens) DSM 1061植酸酶进行突变,突变株D148E活性提高35%,催化效率是野生型的1.5倍,在pH 5.0−8.0范围内活性高于野生型(7.0−20.6 vs. 6.0−15 U/mg),在65 ℃时高达20.6 U/mg,40−75 ℃温度范围内活性增加,且突变株D148E的活性高于野生型(2.5−20.6 vs. 2−15 U/mg)。王陶等[122]对放射型根瘤杆菌(Agrobacterium radiobacter)进行紫外-氯化锂复合诱变,筛选出产植酸酶活性高的菌株,酶活达18.5 U/mL,比原始菌株酶活提高47.7%。
植酸酶对土壤具有一定改良作用,向土壤中施加外源微生物植酸酶制剂或植酸酶生产菌,可有效提高土壤中植酸的分解效率和磷利用率[123] (表 2)。例如,添加微生物植酸酶制剂后,土壤中植酸含量降低了约20.5%,同时有效磷含量提高1.22倍[125]。此外,添加微生物植酸酶可促进土壤植酸磷转化和植物生长。曲博等[126]通过向土壤添加外源微生物植酸酶,发现土壤中活性有机磷含量提高1.73倍,且使黄瓜苗株高、玉米苗干重和株高以及茼蒿苗叶绿素含量分别提高32.4%、74.8%、48.1%和30.6%,3种植物幼苗的生物量与微生物植酸酶的添加量呈显著正相关[127]。因此,微生物植酸酶作为生物菌剂,可提高土壤内源植酸盐的利用效率,减少外源磷肥施用,降低磷流失的环境污染风险,促进农业可持续发展。
5 结论与展望环境磷污染已引起广泛关注,尤其是农业活动过度施肥及动物产生的高磷粪便使大量磷进入土壤,具有潜在的污染土壤和水体风险。无机磷肥施入土壤后极易被土壤吸附或与金属离子形成难溶性络合物或转化为有机磷(植酸盐为主要成分),导致其生物可利用性降低。植酸酶是水解植酸盐的关键酶,在此过程中植酸酶的热稳定性和活性是影响植酸盐水解和磷释放的重要因素。因此,通过阐述土壤中微生物植酸酶活性的影响因素(温度、pH、土壤吸附、钙含量及钙磷比、底物浓度、含量和有效性等),重点综述提高植酸酶活性和稳定性的方法技术(纳米材料负载植酸酶、提高植酸酶pH适应范围、定点突变等)及应用效率。
植酸酶的适当选择是其应用成功的关键,微生物植酸酶在实际应用中仍具有一定局限性,为此,可加强几方面研究:(1) 了解微生物植酸酶的来源,致力于筛选出热稳定性好、活性高的产植酸酶微生物;(2) 理解土壤中植酸酶活性的影响因素,进而寻求方法提高植酸酶在土壤中的活性;(3) 调节植酸酶最适pH范围、提高植酸酶热稳定性、将植酸酶负载于纳米材料和基因工程改造等改善植酸酶性质;(4) 研究植酸酶的作用机制,进一步为微生物植酸酶应用于农业生产及提高土壤难利用态植酸磷的利用效率提供理论基础和技术参考,对降低农业生产成本、减少环境磷污染、控制过量磷肥施用导致的潜在面源污染及水体富营养化风险具有重要的现实意义。
| [1] |
HALLAMA M, PEKRUN C, LAMBERS H, KANDELER E. Hidden miners–the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems[J]. Plant and Soil, 2019, 434(1/2): 7-45. |
| [2] |
RICHARDSON AE, LYNCH JP, RYAN PR, DELHAIZE E, SMITH FA, SMITH SE, HARVEY PR, RYAN MH, VENEKLAAS EJ, LAMBERS H, OBERSON A, CULVENOR RA, SIMPSON RJ. Plant and microbial strategies to improve the phosphorus efficiency of agriculture[J]. Plant and Soil, 2011, 349(1): 121-156. |
| [3] |
QUIQUAMPOIX H, MOUSAIN D. Enzymatic Hydrolysis of Organic Phosphorus[M]. Organic Phosphorus in the Environment. UK: CABI Publishing, 2004: 89-112.
|
| [4] |
RAHMAN MKU, WANG XX, GAO DM, ZHOU XG, WU FZ. Root exudates increase phosphorus availability in the tomato/potato onion intercropping system[J]. Plant and Soil, 2021, 464(1): 45-62. |
| [5] |
STEFFENS D, LEPPIN T, LUSCHIN-EBENGREUTH N, YANG ZM, SCHUBERT S. Organic soil phosphorus considerably contributes to plant nutrition but is neglected by routine soil-testing methods[J]. Journal of Plant Nutrition and Soil Science, 2010, 173(5): 765-771. DOI:10.1002/jpln.201000079 |
| [6] |
LIU X, FENG HY, FU JW, SUN D, CAO Y, CHEN YS, XIANG P, LIU YG, MA LQ. Phytate promoted arsenic uptake and growth in arsenic-hyperaccumulator Pteris vittata by upregulating phosphorus transporters[J]. Environmental Pollution, 2018, 241: 240-246. DOI:10.1016/j.envpol.2018.05.054 |
| [7] |
LIU X. Arsenic resistant bacteria and root organic acid promoted plant growth and arsenic uptake in As-hyperaccumulator Pteris vittata[D]. Nanjing: Doctoral Dissertation of Nanjing University, 2017 (in Chinese). 刘雪. 抗砷细菌及根系有机酸对砷超富集植物蜈蚣草促生及吸砷机理研究[D]. 南京: 南京大学博士学位论文, 2017. |
| [8] |
LI YF, LUO AC, WU LH, WEI XH. Difference in P utilization from organic phosphate between two rice genotypes and its relations with root-secreted acid phosphatase activity[J]. Chinese Journal of Applied Ecology, 2009, 20(5): 1072-1078. (in Chinese) 李永夫, 罗安程, 吴良欢, 魏兴华. 两个基因型水稻利用有机磷的差异及其与根系分泌酸性磷酸酶活性的关系[J]. 应用生态学报, 2009, 20(5): 1072-1078. DOI:10.13287/j.1001-9332.2009.0171 |
| [9] |
LIU X, FU JW, GUAN DX, CAO Y, LUO J, RATHINASABAPATHI B, CHEN YS, MA LQ. Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata[J]. Environmental Science & Technology, 2016, 50(17): 9070-9077. |
| [10] |
PUPPALA KR, NAIK T, SHAIK A, DASTAGER S, KUMAR VR, KHIRE J, DHARNE M. Evaluation of Candida tropicalis (NCIM 3321) extracellular phytase having plant growth promoting potential and process development[J]. Biocatalysis and Agricultural Biotechnology, 2018, 13: 225-235. DOI:10.1016/j.bcab.2017.12.013 |
| [11] |
SAXENA A, VERMA M, SINGH B, SANGWAN P, YADAV AN, DHALIWAL HS, KUMAR V. Characteristics of an acidic phytase from Aspergillus aculeatus APF1 for dephytinization of biofortified wheat genotypes[J]. Applied Biochemistry and Biotechnology, 2020, 191(2): 679-694. DOI:10.1007/s12010-019-03205-9 |
| [12] |
TAN H, WU X, XIE LY, HUANG ZQ, PENG WH, GAN BC. Identification and characterization of a mesophilic phytase highly resilient to high-temperatures from a fungus-garden associated metagenome[J]. Applied Microbiology and Biotechnology, 2016, 100(5): 2225-2241. DOI:10.1007/s00253-015-7097-9 |
| [13] |
XIE ZM, FONG WP, TSANG PWK. Engineering and optimization of phosphate-responsive phytase expression in Pichia pastoris yeast for phytate hydrolysis[J]. Enzyme and Microbial Technology, 2020, 137: 109533. DOI:10.1016/j.enzmictec.2020.109533 |
| [14] |
XU L, ZENG LY, REN L, CHEN W, LIU F, YANG H, YAN RB, CHEN KR, FANG XP. Marker-free lines of phytase-transgenic Brassica napus show enhanced ability to utilize phytate[J]. Plant Cell, Tissue and Organ Culture: PCTOC, 2020, 140(1): 11-22. DOI:10.1007/s11240-019-01706-3 |
| [15] |
ZHANG SP, LIAO SA, YU XY, LU HW, XIAN JA, GUO H, WANG AL, XIE J. Microbial diversity of mangrove sediment in Shenzhen Bay and gene cloning, characterization of an isolated phytase-producing strain of SPC09 B. cereus[J]. Applied Microbiology and Biotechnology, 2015, 99(12): 5339-5350. DOI:10.1007/s00253-015-6405-8 |
| [16] |
SANNI DM, LAWAL OT, ENUJIUGHA VN. Purification and characterization of phytase from Aspergillus fumigatus isolated from African Giant Snail (Achatina fulica)[J]. Biocatalysis and Agricultural Biotechnology, 2019, 17: 225-232. DOI:10.1016/j.bcab.2018.11.017 |
| [17] |
GEORGE TS, SIMPSON RJ, HADOBAS PA, RICHARDSON AE. Expression of a fungal phytase gene in Nicotiana tabacum improves phosphorus nutrition of plants grown in amended soils[J]. Plant Biotechnology Journal, 2005, 3(1): 129-140. DOI:10.1111/j.1467-7652.2004.00116.x |
| [18] |
AZEEM M, RIAZ A, CHAUDHARY AN, HAYAT R, HUSSAIN Q, TAHIR MI, IMRAN M. Microbial phytase activity and their role in organic P mineralization[J]. Archives of Agronomy and Soil Science, 2015, 61(6): 751-766. DOI:10.1080/03650340.2014.963796 |
| [19] |
WANG SB, GAI YM, GONG DC, TU X, ZHANG DW. Heterologous expression and optimization of Debaryomyces castellii phytase gene in Pichia pastoris[J]. Microbiology China, 2021, 48(10): 3421-3431. (in Chinese) 望松柏, 盖园明, 龚大春, 涂璇, 张大伟. 卡氏德巴利酵母植酸酶在毕赤酵母中的异源表达及优化[J]. 微生物学通报, 2021, 48(10): 3421-3431. DOI:10.13344/j.microbiol.china.201209 |
| [20] |
GREINER R. Activity of Escherichia coli, Aspergillus niger, and rye phytase toward partially phosphorylated myo-inositol phosphates[J]. Journal of Agricultural and Food Chemistry, 2017, 65(44): 9603-9607. DOI:10.1021/acs.jafc.7b03897 |
| [21] |
GUIMARÃES LHS, PEIXOTO-NOGUEIRA SC, MICHELIN M, RIZZATTI ACS, SANDRIM VC, ZANOELO FF, AQUINO ACMM, JUNIOR AB, de LOURDES TM POLIZELI M. Screening of filamentous fungi for production of enzymes of biotechnological interest[J]. Brazilian Journal of Microbiology, 2006, 37(4): 474-480. DOI:10.1590/S1517-83822006000400014 |
| [22] |
DVOŘÁKOVÁ J. Phytase: sources, preparation and exploitation[J]. Folia Microbiologica, 1998, 43(4): 323-338. DOI:10.1007/BF02818571 |
| [23] |
YANG YL, SHA L, FANG JT. Research and application of phytase from microbe[J]. Wuyi Science Journal, 2007, 23: 191-195. (in Chinese) 杨燕凌, 沙莉, 方金铜. 微生物植酸酶的研究与应用[J]. 武夷科学, 2007, 23: 191-195. DOI:10.15914/j.cnki.wykx.2007.00.038 |
| [24] |
CHEN H, WANG HN, XIE J. Purifiaction and characteristics of the phytase from A. niger N25[J]. Journal of Sichuan Agricultural University, 2001, 19(1): 77-79. (in Chinese) 陈惠, 王红宁, 谢晶. 黑曲霉N25植酸酶的分离纯化及酶学性质研究[J]. 四川农业大学学报, 2001, 19(1): 77-79. DOI:10.16036/j.issn.1000-2650.2001.01.020 |
| [25] |
KONIETZNY U, GREINER R. Molecular and catalytic properties of phytate-degrading enzymes (phytases)[J]. International Journal of Food Science and Technology, 2002, 37(7): 791-812. DOI:10.1046/j.1365-2621.2002.00617.x |
| [26] |
CHADHA BS, HARMEET G, MANDEEP M, SAINI HS, SINGH N. Phytase production by the thermophilic fungus Rhizomucor pusillus[J]. World Journal of Microbiology and Biotechnology, 2004, 20(1): 105-109. DOI:10.1023/B:WIBI.0000013319.13348.0a |
| [27] |
GREINER R, SAJIDAN. Production of D-myo-inositol(1, 2, 4, 5, 6)pentakisphosphate using alginate-entrapped recombinant Pantoea agglomerans glucose-1-phosphatase[J]. Brazilian Archives of Biology and Technology, 2008, 51(2): 235-246. DOI:10.1590/S1516-89132008000200002 |
| [28] |
BANDYOPADHYAY D, DAS K, SEN SK. Purification of thermo and acid tolerant extracellular phytase from a new soil isolate of Amycolatopsis vancoresmycina S-12[J]. Biocatalysis and Agricultural Biotechnology, 2017, 11: 302-306. DOI:10.1016/j.bcab.2017.08.002 |
| [29] |
HUANG WQ. Research on the enzymatic characterization and structure-function relationship of lipase AFLB from Aspergillus fumigatus[D]. Guangzhou: Doctoral Dissertation of South China University of Technology, 2019 (in Chinese). 黄伟谦. 烟曲霉脂肪酶AFLB的酶学性质及其结构功能关系研究[D]. 广州: 华南理工大学博士学位论文, 2019. |
| [30] |
RAGON M, AUMELAS A, CHEMARDIN P, GALVEZ S, MOULIN G, BOZE H. Complete hydrolysis of myo-inositol hexakisphosphate by a novel phytase from Debaryomyces castellii CBS 2923[J]. Applied Microbiology and Biotechnology, 2008, 78(1): 47-53. DOI:10.1007/s00253-007-1275-3 |
| [31] |
SAJIDAN A, FAROUK A, GREINER R, JUNGBLUT P, MÜLLER EC, BORRISS R. Molecular and physiological characterisation of a 3-phytase from soil bacterium Klebsiella sp. ASR1[J]. Applied Microbiology and Biotechnology, 2004, 65(1): 110-118. |
| [32] |
LI XL, YANG HT, HU JD, WU YZ, LI JS, REN Y. Diversity and classification of phytases[J]. Microbiology China, 2010, 37(5): 738-747. (in Chinese) 李晓龙, 杨合同, 扈进冬, 吴远征, 李纪顺, 任艳. 植酸酶的多样性及其分类[J]. 微生物学通报, 2010, 37(5): 738-747. DOI:10.13344/j.microbiol.china.2010.05.001 |
| [33] |
PUHL AA, GREINER R, SELINGER LB. A protein tyrosine phosphatase-like inositol polyphosphatase from Selenomonas ruminantium subsp. lactilytica has specificity for the 5-phosphate of myo-inositol hexakisphosphate[J]. The International Journal of Biochemistry & Cell Biology, 2008, 40(10): 2053-2064. |
| [34] |
LASSEN SF, BREINHOLT J, ØSTERGAARD PR, BRUGGER R, BISCHOFF A, WYSS M, FUGLSANG CC. Expression, gene cloning, and characterization of five novel phytases from four basidiomycete fungi: Peniophora lycii, Agrocybe pediades, a Ceriporia sp., and Trametes pubescens[J]. Applied and Environmental Microbiology, 2001, 67(10): 4701-4707. DOI:10.1128/AEM.67.10.4701-4707.2001 |
| [35] |
PUHL AA, GREINER R, SELINGER LB. Stereospecificity of myo-inositol hexakisphosphate hydrolysis by a protein tyrosine phosphatase-like inositol polyphosphatase from Megasphaera elsdenii[J]. Applied Microbiology and Biotechnology, 2009, 82(1): 95-103. DOI:10.1007/s00253-008-1734-5 |
| [36] |
BALWANI I, CHAKRAVARTY K, GAUR S. Role of phytase producing microorganisms towards agricultural sustainability[J]. Biocatalysis and Agricultural Biotechnology, 2017, 12: 23-29. DOI:10.1016/j.bcab.2017.08.010 |
| [37] |
LUO HY, HUANG HQ, YANG PL, WANG YR, YUAN TZ, WU NF, YAO B, FAN YL. A novel phytase appA from Citrobacter amalonaticus CGMCC 1696: gene cloning and overexpression in Pichia pastoris[J]. Current Microbiology, 2007, 55(3): 185-192. DOI:10.1007/s00284-006-0586-4 |
| [38] |
LI XY, LIU ZQ, CHI ZM, LI J, WANG XH. Molecular cloning, characterization, and expression of the phytase gene from marine yeast Kodamaea ohmeri BG3[J]. Mycological Research, 2009, 113(1): 24-32. DOI:10.1016/j.mycres.2008.07.003 |
| [39] |
FUGTHONG A, BOONYAPAKRON K, SORNLEK W, TANAPONGPIPAT S, EURWILAICHITR L, POOTANAKIT K. Biochemical characterization and in vitro digestibility assay of Eupenicillium parvum (BCC17694) phytase expressed in Pichia pastoris[J]. Protein Expression and Purification, 2010, 70(1): 60-67. DOI:10.1016/j.pep.2009.10.001 |
| [40] |
MITCHELL DB, VOGEL K, WEIMANN BJ, PASAMONTES L, van LOON APGM. The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila[J]. Microbiology: Reading, England, 1997, 143(Pt 1): 245-252. |
| [41] |
XU W, SHAO R, WANG ZP, YAN XH. Improving the neutral phytase activity from Bacillus amyloliquefaciens DSM 1061 by site-directed mutagenesis[J]. Applied Biochemistry and Biotechnology, 2015, 175(6): 3184-3194. DOI:10.1007/s12010-015-1495-4 |
| [42] |
HA NC, OH BC, SHIN S, KIM HJ, OH TK, KIM YO, CHOI KY, OH BH. Crystal structures of a novel, thermostable phytase in partially and fully calcium-loaded states[J]. Nature Structural Biology, 2000, 7(2): 147-153. DOI:10.1038/72421 |
| [43] |
SHIN S, HA NC, OH BC, OH TK, OH BH. Enzyme mechanism and catalytic property of β propeller phytase[J]. Structure, 2001, 9(9): 851-858. DOI:10.1016/S0969-2126(01)00637-2 |
| [44] |
HONG CY, CHENG KJ, TSENG TH, WANG CS, LIU LF, YU SM. Production of two highly active bacterial phytases with broad pH optima in germinated transgenic rice seeds[J]. Transgenic Research, 2004, 13(1): 29-39. DOI:10.1023/B:TRAG.0000017158.96765.67 |
| [45] |
CHU HM, GUO RT, LIN TW, CHOU CC, SHR HL, LAI HL, TANG TY, CHENG KJ, SELINGER BL, WANG AHJ. Structures of Selenomonas ruminantium phytase in complex with persulfated phytate: DSP phytase fold and mechanism for sequential substrate hydrolysis[J]. Structure: London, England: 1993, 2004, 12(11): 2015-2024. |
| [46] |
GRUNINGER RJ, SELINGER LB, MOSIMANN SC. Effect of ionic strength and oxidation on the P-loop conformation of the protein tyrosine phosphatase-like phytase, PhyAsr[J]. The FEBS Journal, 2008, 275(15): 3783-3792. DOI:10.1111/j.1742-4658.2008.06524.x |
| [47] |
LUNG SC, LEUNG A, KUANG R, WANG Y, LEUNG P, LIM BL. Phytase activity in tobacco (Nicotiana tabacum) root exudates is exhibited by a purple acid phosphatase[J]. Phytochemistry, 2008, 69(2): 365-373. DOI:10.1016/j.phytochem.2007.06.036 |
| [48] |
KUANG RB, CHAN KH, YEUNG E, LIM BL. Molecular and biochemical characterization of AtPAP15, a purple acid phosphatase with phytase activity, in Arabidopsis[J]. Plant Physiology, 2009, 151(1): 199-209. DOI:10.1104/pp.109.143180 |
| [49] |
CHENG CW, LIM BL. Beta-propeller phytases in the aquatic environment[J]. Archives of Microbiology, 2006, 185(1): 1-13. DOI:10.1007/s00203-005-0080-6 |
| [50] |
SCHENK G, GUDDAT LW, GE Y, CARRINGTON LE, HUME DA, HAMILTON S, de JERSEY J. Identification of mammalian-like purple acid phosphatases in a wide range of plants[J]. Gene, 2000, 250(1/2): 117-125. |
| [51] |
REDDY CS, ACHARY VMM, MANNA M, SINGH J, KAUL T, REDDY MK. Isolation and molecular characterization of thermostable phytase from Bacillus subtilis (BSPhyARRMK33)[J]. Applied Biochemistry and Biotechnology, 2015, 175(6): 3058-3067. DOI:10.1007/s12010-015-1487-4 |
| [52] |
JORQUERA M, MARTÍNEZ O, MARUYAMA F, MARSCHNER P, de la LUZ MORA M. Current and future biotechnological applications of bacterial phytases and phytase-producing bacteria[J]. Microbes and Environments, 2008, 23(3): 182-191. DOI:10.1264/jsme2.23.182 |
| [53] |
WANG X, DU J, ZHANG ZY, FU YJ, WANG WM, LIANG AH. A rational design to enhance the resistance of Escherichia coli phytase appA to trypsin[J]. Applied Microbiology and Biotechnology, 2018, 102(22): 9647-9656. DOI:10.1007/s00253-018-9327-4 |
| [54] |
MENG X, LUO JR, DERSJANT-LI Y. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors[J]. Animal Science Abroad: Pigs and Poultry, 2021, 41(4): 124-129. (in Chinese) 孟霞, 罗静如, DERSJANT-LI Y. 非反刍动物营养中的植酸酶: 胃肠道植酸酶活性及其影响因素[J]. 国外畜牧学: 猪与禽, 2021, 41(4): 124-129. |
| [55] |
BOHN L, MEYER AS, RASMUSSEN SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding[J]. Journal of Zhejiang University Science B, 2008, 9(3): 165-191. DOI:10.1631/jzus.B0710640 |
| [56] |
SINGH B, KUNZE G, SATYANARAYANA T. Developments in biochemical aspects and biotechnological applications of microbial phytases[J]. Biotechnol Mol Biol Rev, 2011, 6(3): 69-87. |
| [57] |
RICHARDSON AE. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants[J]. Functional Plant Biology, 2001, 28(9): 897-906. DOI:10.1071/PP01093 |
| [58] |
LEI XG, PORRES JM. Phytase enzymology, applications, and biotechnology[J]. Biotechnology Letters, 2003, 25(21): 1787-1794. DOI:10.1023/A:1026224101580 |
| [59] |
YU HL. Critical control points for the determination of feed phytase activity[J]. Feed Review, 2019(8): 38-40, 44. (in Chinese) 于洪莲. 饲用植酸酶活性测定关键控制点[J]. 饲料博览, 2019(8): 38-40, 44. |
| [60] |
SHI BM, SHAN AS. Determination of microbial phytase activity in vitro[J]. Heilongjinag Journal of Animal Science and Veterinary Medicine, 2000(2): 15-16. (in Chinese) 石宝明, 单安山. 微生物植酸酶在体外条件下活性的测定[J]. 黑龙江畜牧兽医, 2000(2): 15-16. DOI:10.13881/j.cnki.hljxmsy.2000.02.013 |
| [61] |
TIAN YS, PENG RH, XU J, ZHAO W, GAO F, FU XY, XIONG AS, YAO QH. Mutations in two amino acids in phyI1s from Aspergillus niger 113 improve its phytase activity[J]. World Journal of Microbiology and Biotechnology, 2010, 26(5): 903-907. DOI:10.1007/s11274-009-0251-8 |
| [62] |
QI YX, CHEN YL. Mechanism of phytase and factors affecting phytase activity[J]. Feed Review, 2004(7): 10-12. (in Chinese) 祁艳霞, 陈玉林. 植酸酶的作用机理及影响植酸酶活性的因素[J]. 饲料博览, 2004(7): 10-12. |
| [63] |
SHAN AS, WANG A, XU QY, SHI BM, DU J, SONG JC, MA X, WANG LJ. Study on properties in vitro and roles of phytase in poultry 1. The properties of phytase of microbial and plant origins and affecting factors in vitro[J]. Journal of Northeast Agricultural University, 2001, 32(4): 345-351. (in Chinese) 单安山, 王安, 徐奇友, 石宝明, 杜鹃, 宋金彩, 马玺, 王丽娟. 植酸酶的特性及其在家禽饲粮中应用的研究1.微生物植酸酶、植物植酸酶特性的测定与影响因素的研究[J]. 东北农业大学学报, 2001, 32(4): 345-351. |
| [64] |
WANG XY, UPATHAM S, PANBANGRED W, ISARANGKUL D, WIYAKRUTTA S, MEEVOOTISOM V. Purification, characterization, gene cloning and sequence analysis of a phytase from Klebsiella pneumoniae subsp. pneumoniae XY-5[J]. Science Asia, 2004, 30: 383-390. DOI:10.2306/scienceasia1513-1874.2004.30.383 |
| [65] |
PUPPALA KR, BHAVSAR K, SONALKAR V, KHIRE JM, DHARNE MS. Characterization of novel acidic and thermostable phytase secreting Streptomyces sp. (NCIM 5533) for plant growth promoting characteristics[J]. Biocatalysis and Agricultural Biotechnology, 2019, 18: 101020. DOI:10.1016/j.bcab.2019.101020 |
| [66] |
LI J, LIU ZB. Secretive expression of heat-stable phytase of Aspergillus fumigatus in Aspergillus niger[J]. Journal of Microbiology, 2007, 27(3): 61-64. (in Chinese) 李佳, 刘钟滨. 烟曲霉菌热稳定性植酸酶在黑曲霉菌中的分泌性表达[J]. 微生物学杂志, 2007, 27(3): 61-64. |
| [67] |
YIN DS, ZHOU YJ, PAN B. Study on kinetic properties of microbial phytase[J]. Journal of Nanchang College, 2010, 25(1): 166-168. (in Chinese) 尹德胜, 周永江, 潘斌. 微生物植酸酶动力学性质的研究[J]. 南昌高专学报, 2010, 25(1): 166-168. |
| [68] |
WYSS M, PASAMONTES L, RÉMY R, KOHLER J, KUSZNIR E, GADIENT M, MÜLLER F, APGM VL. Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus phytase, A. niger phytase, and A. niger pH 2.5 acid phosphatase[J]. Applied and Environmental Microbiology, 1998, 64(11): 4446-4451. DOI:10.1128/AEM.64.11.4446-4451.1998 |
| [69] |
XING ZL, CHEN HY, XIE F, WU ZR. Progress on phytase and its thermostability[J]. Progress in Biotechnology, 2003, 23(5): 31-35. (in Chinese) 邢自力, 陈华友, 谢芳, 吴自荣. 植酸酶及其热稳定性研究进展[J]. 中国生物工程杂志, 2003, 23(5): 31-35. |
| [70] |
HUANG Y, HU Y, MAO XW, HUAN ZL, LI JL. Study on the application of phytase in aquatic products[J]. Hunan Feed, 2010(5): 26-29. (in Chinese) 黄云, 胡毅, 毛小伟, 郇志利, 李金龙. 水产饲料中植酸酶的应用研究[J]. 湖南饲料, 2010(5): 26-29. |
| [71] |
SUN GQ. Study on nutrient utilization rate of maggot fecal nutrient enrichment and maggot[D]. Shenyang: Master's Thesis of Shenyang Agricultural University, 2016 (in Chinese). 孙国庆. 植酸酶对不同钙磷含量母猪日粮中养分表观消化率和断奶窝重的影响[D]. 沈阳: 沈阳农业大学硕士学位论文, 2016. |
| [72] |
GREINER R. Purification and properties of a phytate-degrading enzyme from Pantoea agglomerans[J]. The Protein Journal, 2004, 23(8): 567-576. DOI:10.1007/s10930-004-7883-1 |
| [73] |
KEDI B, ABADIE J, SEI J, QUIQUAMPOIX H, STAUNTON S. Diversity of adsorption affinity and catalytic activity of fungal phosphatases adsorbed on some tropical soils[J]. Soil Biology and Biochemistry, 2013, 56: 13-20. DOI:10.1016/j.soilbio.2012.02.006 |
| [74] |
GEORGE TS, RICHARDSON AE, SIMPSON RJ. Behaviour of plant-derived extracellular phytase upon addition to soil[J]. Soil Biology and Biochemistry, 2005, 37(5): 977-988. DOI:10.1016/j.soilbio.2004.10.016 |
| [75] |
MEZELI MM, MENEZES-BLACKBURN D, GEORGE TS, GILES CD, NEILSON R, HAYGARTH PM. Effect of citrate on Aspergillus niger phytase adsorption and catalytic activity in soil[J]. Geoderma, 2017, 305: 346-353. DOI:10.1016/j.geoderma.2017.06.015 |
| [76] |
GIAVENO C, CELI L, RICHARDSON AE, SIMPSON RJ, BARBERIS E. Interaction of phytases with minerals and availability of substrate affect the hydrolysis of inositol phosphates[J]. Soil Biology and Biochemistry, 2010, 42(3): 491-498. DOI:10.1016/j.soilbio.2009.12.002 |
| [77] |
GEORGE TS, QUIQUAMPOIX H, SIMPSON RJ, RICHARDSON AE. Interactions between Phytases and Soil Constituents: Implications for the Hydrolysis of Inositol Phosphates[M]. Inositol Phosphates: Linking Agriculture and the Environment. UK: CABI, 2006: 221-241.
|
| [78] |
LEPRINCE F, QUIQUAMPOIX H. Extracellular enzyme activity in soil: effect of pH and ionic strength on the interaction with montmorillonite of two acid phosphatases secreted by the ectomycorrhizal fungus Hebeloma cylindrosporum[J]. European Journal of Soil Science, 1996, 47(4): 511-522. DOI:10.1111/j.1365-2389.1996.tb01851.x |
| [79] |
TURNER BL, PAPHÁZY MJ, HAYGARTH PM, MCKELVIE ID. Inositol phosphates in the environment[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2002, 357(1420): 449-469. DOI:10.1098/rstb.2001.0837 |
| [80] |
ZHOU T. The mechanism of phytic acid dgradation mediated by polyamines and Ca2+ in mung bean sprouts[D]. Nanjing: Doctoral Dissertation of Nanjing Agricultural University, 2018 (in Chinese). 周婷. 多胺和Ca2+对绿豆芽菜植酸降解的作用机制[D]. 南京: 南京农业大学博士学位论文, 2018. |
| [81] |
ZHANG ZJ, YANG J, XIE PJ, GAO YP, BAI J, ZHANG C, LIU L, WANG Q, GAO XW. Characterization of a thermostable phytase from Bacillus licheniformis WHU and further stabilization of the enzyme through disulfide bond engineering[J]. Enzyme and Microbial Technology, 2020, 142: 109679. DOI:10.1016/j.enzmictec.2020.109679 |
| [82] |
CHENG HN, MO XT, CHEN Y, TAN HB, ZHOU X, XIA LQ. Studies on screening of strains producing phytase and conditions of producing phytase[J]. Life Science Research, 2002, 6(4): 343-346. (in Chinese) 程海娜, 莫湘涛, 陈宇, 谭海滨, 周雄, 夏立秋. 产植酸酶菌株的筛选及产酶条件的研究[J]. 生命科学研究, 2002, 6(4): 343-346. |
| [83] |
TAMIM NM, ANGEL R, CHRISTMAN M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens[J]. Poultry Science, 2004, 83(8): 1358-1367. |
| [84] |
DERSJANT-LI Y, AWATI A, SCHULZE H, PARTRIDGE G. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors[J]. Journal of the Science of Food and Agriculture, 2015, 95(5): 878-896. |
| [85] |
QIAN H, KORNEGAY ET, DENBOW DM. Phosphorus equivalence of microbial phytase in turkey diets as influenced by calcium to phosphorus ratios and phosphorus levels[J]. Poultry Science, 1996, 75(1): 69-81. |
| [86] |
OU XQ, MAO JJ. Study on the application of feed phytase[J]. Feed Industry, 2005, 26(12): 10-13. (in Chinese) 欧秀琼, 毛建军. 饲用植酸酶的应用研究[J]. 饲料工业, 2005, 26(12): 10-13. |
| [87] |
HU XF. Study on the optimum ratio of Ca and P in supplemental phytase diets of growing pigs[D]. Zhengzhou: Master's Thesis of Henan Agricultural University, 2005 (in Chinese). 胡骁飞. 生长猪应用植酸酶日粮适宜钙磷比的研究[D]. 郑州: 河南农业大学硕士学位论文, 2005. |
| [88] |
ZHANG P. Preparation and quality evaluation of phytase pellets from Aspergillus niger[D]. Harbin: Master's Thesis of Helongjiang University, 2010 (in Chinese). 张萍. 黑曲霉植酸酶微丸的制备和质量评价[D]. 哈尔滨: 黑龙江大学硕士学位论文, 2010. |
| [89] |
GUO HC. Research on the catalytic activity of phytase[J]. Journal of Anhui Agricultural Sciences, 2010, 38(8): 3889-3890, 3893. (in Chinese) 郭会灿. 植酸酶催化活性的研究[J]. 安徽农业科学, 2010, 38(8): 3889-3890, 3893. |
| [90] |
MENEZES-BLACKBURN D, JORQUERA MA, GREINER R, GIANFREDA L, de la LUZ MORA M. Phytases and phytase-labile organic phosphorus in manures and soils[J]. Critical Reviews in Environmental Science and Technology, 2013, 43(9): 916-954. |
| [91] |
MARTIN M, CELI L, BARBERIS E. Desorption and plant availability of myo-inositol hexaphosphate adsorbed on goethite[J]. Soil Science, 2004, 169(2): 115-124. |
| [92] |
JOHNSON BB, QUILL E, ANGOVE MJ. An investigation of the mode of sorption of inositol hexaphosphate to goethite[J]. Journal of Colloid and Interface Science, 2012, 367(1): 436-442. |
| [93] |
YAN YP, WAN B, LIU F, TAN WF, LIU MM, FENG XH. Adsorption-desorption of myo-inositol hexakisphosphate on hematite[J]. Soil Science, 2014, 179(10/11): 476-485. |
| [94] |
CHEN A, ARAI Y. Functional group specific phytic acid adsorption at the ferrihydrite-water interface[J]. Environmental Science & Technology, 2019, 53(14): 8205-8215. |
| [95] |
YAN YP, LI W, YANG J, ZHENG AM, LIU F, FENG XH, SPARKS DL. Mechanism of myo-inositol hexakisphosphate sorption on amorphous aluminum hydroxide: spectroscopic evidence for rapid surface precipitation[J]. Environmental Science & Technology, 2014, 48(12): 6735-6742. |
| [96] |
CELI L, BARBERIS E. Abiotic Stabilization of Organic Phosphorus in the Environment[M]. Organic Phosphorus in the Environment. UK: CABI Publishing, 2004: 113-132.
|
| [97] |
CREA F, de STEFANO C, MILEA D, SAMMARTANO S. Formation and stability of phytate complexes in solution[J]. Coordination Chemistry Reviews, 2008, 252(10/11): 1108-1120. |
| [98] |
BERRY DF, SHANG C, WALTHAM SAJDAK CA, ZELAZNY LW. Measurement of phytase activity using tethered phytic acid as an artificial substrate: methods development[J]. Soil Biology and Biochemistry, 2007, 39(1): 361-367. |
| [99] |
LIU X, HAN R, CAO Y, TURNER BL, MA LQ. Enhancing phytate availability in soils and phytate-P acquisition by plants: A review[J]. Environmental Science & Technology, 2022, 56(13): 9196-9219. |
| [100] |
XU SW, CHEN A, ARAI Y. Solution 31P NMR investigation of inositol hexakisphosphate surface complexes at the amorphous aluminum oxyhydroxide-water interface[J]. Environmental Science & Technology, 2021, 55(21): 14628-14638. |
| [101] |
GERKE J. Humic (organic matter)-Al(Fe)-phosphate complexes: an underestimated phosphate form in soils and source of plant-available phosphate[J]. Soil Science, 2010, 175(9): 417-425. |
| [102] |
QI ML, HE K, HUANG ZN, SHAHBAZIAN-YASSAR R, XIAO GY, LU YP, SHOKUHFAR T. Hydroxyapatite fibers: a review of synthesis methods[J]. JOM, 2017, 69(8): 1354-1360. |
| [103] |
COUTINHO TC, TARDIOLI PW, FARINAS CS. Phytase immobilization on hydroxyapatite nanoparticles improves its properties for use in animal feed[J]. Applied Biochemistry and Biotechnology, 2020, 190(1): 270-292. |
| [104] |
WANG K. Isolation of phytase-producing microbes in the soils of Tibetan Plateau and the phytase proferties[D]. Lanzhou: Master's Thesis of Lanzhou Jiaotong University, 2016 (in Chinese). 王凯. 青藏高原土壤中产植酸酵微生物的筛选及酶学特性研究[D]. 兰州: 兰州交通大学硕士学位论文, 2016. |
| [105] |
DUTTA N, RAJ D, BISWAS N, MALLICK M, OMESH S. Nanoparticle assisted activity optimization and characterization of a bacterial phytase immobilized on single layer graphene oxide[J]. Biocatalysis and Agricultural Biotechnology, 2017, 9: 240-247. |
| [106] |
QIAN H. Preparation, characterization and in vitro digestion of phytase nanoparticle based on chitosan embedding[D]. Yangling: Master's Thesis of Northwest A & F University, 2021 (in Chinese). 钱浩. 基于壳聚糖包埋的植酸酶纳米粒制备、特性及体外消化研究[D]. 杨凌: 西北农林科技大学硕士学位论文, 2021. |
| [107] |
HARATI J, RANAEI SIADAT SO, TAGHAVIAN H, KABOLI S, KHORSHIDI S. Improvement in biochemical characteristics of glycosylated phytase through immobilization on nanofibers[J]. Biocatalysis and Agricultural Biotechnology, 2017, 12: 96-103. |
| [108] |
WANG CB. Study on immobilization conditions of phytase[J]. Heilongjiang Agricultural Sciences, 2014(5): 99-102. (in Chinese) 王成波. 植酸酶固定化条件的研究[J]. 黑龙江农业科学, 2014(5): 99-102. |
| [109] |
BEI JL, CHEN Z, FU J, JIANG ZY, WANG JW, WANG XZ. Structure-based fragment shuffling of two fungal phytases for combination of desirable properties[J]. Journal of Biotechnology, 2009, 139(2): 186-193. |
| [110] |
LI C, LIN Y, HUANG YY, LIU XX, LIANG SL. Citrobacter amalonaticus phytase on the cell surface of Pichia pastoris exhibits high pH stability as a promising potential feed supplement[J]. PLoS One, 2014, 9(12): e114728. |
| [111] |
SHIVANGE AV, DENNIG A, SCHWANEBERG U. Multi-site saturation by OmniChange yields a pH- and thermally improved phytase[J]. Journal of Biotechnology, 2014, 170: 68-72. |
| [112] |
RODRIGUEZ E, WOOD ZA, KARPLUS PA, LEI XG. Site-directed mutagenesis improves catalytic efficiency and thermostability of Escherichia coli pH 2.5 acid phosphatase/phytase expressed in Pichia pastoris[J]. Archives of Biochemistry and Biophysics, 2000, 382(1): 105-112. |
| [113] |
ZHU WH, QIAO DR, HUANG M, YANG G, XU H, CAO Y. Modifying thermostability of appA from Escherichia coli[J]. Current Microbiology, 2010, 61(4): 267-273. |
| [114] |
KIM MS, LEI XG. Enhancing thermostability of Escherichia coli phytase AppA2 by error-prone PCR[J]. Applied Microbiology and Biotechnology, 2008, 79(1): 69-75. |
| [115] |
KIM MS, WEAVER JD, LEI XG. Assembly of mutations for improving thermostability of Escherichia coli AppA2 phytase[J]. Applied Microbiology and Biotechnology, 2008, 79(5): 751-758. |
| [116] |
FEI BJ, CAO Y, XU H, LI XR, SONG T, FEI ZA, QIAO DR, CAO Y. AppA C-terminal plays an important role in its thermostability in Escherichia coli[J]. Current Microbiology, 2013, 66(4): 374-378. |
| [117] |
TANG ZZ, JIN WQ, SUN R, LIAO Y, ZHEN TR, CHEN H, WU Q, GOU L, LI CL. Improved thermostability and enzyme activity of a recombinant phyA mutant phytase from Aspergillus niger N25 by directed evolution and site-directed mutagenesis[J]. Enzyme and Microbial Technology, 2018, 108: 74-81. |
| [118] |
HESAMPOUR A, SIADAT SER, MALBOOBI MA, MOHANDESI N, ARAB SS, GHAHREMANPOUR MM. Enhancement of thermostability and kinetic efficiency of Aspergillus niger PhyA phytase by site-directed mutagenesis[J]. Applied Biochemistry and Biotechnology, 2015, 175(5): 2528-2541. |
| [119] |
LIAO Y. Improvement in the thermostability and enzyme activity of a recombinant phya mutant phytase from Aspergillus niger N25by directed evolution[D]. Yaan: Master's Thesis of Sichuan Agricultural University, 2012 (in Chinese). 廖燕. 定向进化技术提高重组突变黑曲霉N25植酸酶酶活及热稳定性的研究[D]. 雅安: 四川农业大学硕士学位论文, 2012. |
| [120] |
YE M, SHEN JZ, YE M, SUN HJ, LIU GQ. Breeding of high-yield phytase strains and optimization of its fermentation conditions[J]. Food Science and Technology, 2009, 34(4): 10-13. (in Chinese) 叶明, 沈君子, 叶敏, 孙汉巨, 刘国庆. 高产植酸酶菌株的选育及发酵条件优化[J]. 食品科技, 2009, 34(4): 10-13. |
| [121] |
CHEN HG, MIAO XX, ZHANG SM, JIA XC. Mutation breeding of phytase-producing strains[J]. Microbiology, 1997, 24(5): 272-274. (in Chinese) 陈红歌, 苗雪霞, 张世敏, 贾新成. 植酸酶高产菌株的诱变选育[J]. 微生物学通报, 1997, 24(5): 272-274. |
| [122] |
WANG T, LI W, YIN L, LIU Y. Mutation breeding of neutral phytase-producing strain using both UV light and lithium chloride[J]. Food Science, 2012, 33(13): 217-220. (in Chinese) 王陶, 李文, 尹龙, 刘洋. 紫外-氯化锂复合诱变选育中性植酸酶高产菌株[J]. 食品科学, 2012, 33(13): 217-220. |
| [123] |
DING R, CHEN XH, LI BX. Research advances on phytase and prospect of applying soil phytase[J]. Biotechnology Bulletin, 2019, 35(7): 190-195. (in Chinese) 丁锐, 陈旭辉, 李炳学. 植酸酶研究进展及土壤植酸酶应用展望[J]. 生物技术通报, 2019, 35(7): 190-195. |
| [124] |
ZHANG XX, GESSLER N. Phytases and the prospects for their application(review)[J]. Animal Science Abroad: Pigs and Poultry, 2020, 40(10): 73-76. (in Chinese) 张相鑫, GESSLER N. 植酸酶及其应用前景(续2)[J]. 国外畜牧学: 猪与禽, 2020, 40(10): 73-76. |
| [125] |
LU F. Study on the characteristics of dual-domain β-propeller phytase and its application in soil and seedling[D]. Beijing: Doctoral Dissertation of Beijing Forestry University, 2020 (in Chinese). 鲁芳. 双功能域β-折叠桶植酸酶的特性及其在土壤与育苗中的应用研究[D]. 北京: 北京林业大学博士学位论文, 2020. |
| [126] |
QU B, LI M, QI M, ZHU YY, ZHAO T, SUN XJ. Effects of soil organic phosphorus transformation on fertilizing outside source of phytase in Yeyahu wetland[J]. Ecology and Environmental Sciences, 2015, 24(2): 250-254. (in Chinese) 曲博, 李敏, 其美, 朱芸芸, 赵暾, 孙晓建. 外源植酸酶对野鸭湖湿地土壤有机磷转化的影响研究[J]. 生态环境学报, 2015, 24(2): 250-254. |
| [127] |
SU Y, DU WY, LI XX, HUANG SJ, LIU LL. Promoting effects of microbial phytase fertilizer on growth of crops[J]. Journal of Tianjin Normal University (Natural Science Edition), 2014, 34(1): 78-80. (in Chinese) 苏毅, 杜文雅, 李晓晓, 黄世江, 刘丽丽. 植酸酶生物肥料对农作物生长的促进作用[J]. 天津师范大学学报(自然科学版), 2014, 34(1): 78-80. |
 2023, Vol. 50
2023, Vol. 50