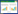扩展功能
文章信息
- 王阳阳, 李佳倩, 朱星宇, 刘璐, 汪光义
- WANG Yangyang, LI Jiaqian, ZHU Xingyu, LIU Lu, WANG Guangyi
- 利用环境胁迫提高破囊壶菌二十二碳六烯酸生产的研究进展
- The enhanced docosahexaenoic acid production by thraustochytrids through environmental stresses: a review
- 微生物学通报, 2023, 50(11): 5150-5171
- Microbiology China, 2023, 50(11): 5150-5171
- DOI: 10.13344/j.microbiol.china.230268
-
文章历史
- 收稿日期: 2023-04-05
- 接受日期: 2023-06-14
- 网络首发日期: 2023-07-21
2. 天津大学青岛海洋技术研究院, 山东 青岛 266200
2. Qingdao Institute of Ocean Technology, Tianjin University, Qingdao 266200, Shandong, China
破囊壶菌可以产生占细胞干重50%以上的脂质,同时具有较强的脂肪酸生物合成能力[1]。破囊壶菌细胞中的多不饱和脂肪酸(polyunsaturated fatty acids, PUFA)主要分布在三酰甘油和细胞膜的磷脂中[2]。一方面,以三酰甘油形式储存的PUFA可以作为能量和碳源储备;另一方面,PUFA作为磷脂的重要组成成分,其含量会影响细胞膜的流动性,并且PUFA的抗氧化作用能够保护细胞膜及膜蛋白免受氧化损伤,从而维持细胞膜的正常功能[3]。二十二碳六烯酸(docosahexaenoic acid, DHA)是破囊壶菌细胞中最主要的PUFA,在总脂肪酸(total fatty acids, TFA)中的含量能够达到40%−50%甚至更高[4-5]。DHA可以促进视网膜、神经系统和大脑的发育,并且在抗炎、抗癌、抗氧化、抗凝血、降血脂和机体免疫调节等方面发挥重要作用,是人体必需的一种ω-3 PUFA,成为生产婴幼儿配方奶粉和各种食品、保健品的原料之一[6-8]。目前人类主要的DHA膳食来源是深海鱼油,由于海洋污染、深海鱼类过度捕捞造成的不可持续性等问题,人们不断寻找新的DHA生产原料[9]。破囊壶菌生长速度快、培养方式简单、发酵条件可操控并且脂质含量高,可以作为绿色且可持续发展的人类膳食DHA替代来源[10]。
破囊壶菌的生长需要适宜的培养条件,包括适宜的碳氮源、微量元素等营养因子,以及温度、盐度、溶氧和pH等环境因子[11-12],其中任何一种因子造成的胁迫条件都会破坏细胞稳态,使细胞的生理和代谢过程发生变化。环境胁迫会诱导破囊壶菌脂质积累,然而这往往以降低生物量为代价,从而导致脂质和DHA产量降低[13]。一直以来,如何利用环境胁迫在促进脂质积累的同时减少生长限制是研究者尝试解决的问题。因此,本文阐述了破囊壶菌在不同环境胁迫因子下生长、脂质积累、脂肪酸组成和DHA生产方面的响应,并在此基础上总结了当前基于环境胁迫因子的分段式发酵策略,以及提高抗氧化能力以缓解氧化应激的多种策略在提高破囊壶菌DHA产量方面的研究现状,最后提出了目前破囊壶菌对环境胁迫响应的分子机制研究和相关的发酵策略存在的问题,以及未来可能的发展方向,以期为破囊壶菌的DHA高效工业化生产提供参考。
1 破囊壶菌概述 1.1 破囊壶菌简介破囊壶菌是一种单细胞真核专性异养原生生物,广泛分布于大洋、河口、红树林地区、大型藻类、浅层沉积物和盐沼等富含有机碎屑和腐烂生物质的生境中,以满足其异养生存的营养需求[14-15]。目前已有近百种破囊壶菌菌株从不同栖息地中被分离鉴定并进行培养与研究[16]。利用现代分子生物学技术,破囊壶菌被归类为生物界(Stramenopila)不等毛门(Heterokonta)网粘菌纲(Labyrinthulomycetes)破囊壶菌目(Thraustochytriales)破囊壶菌科(Thraustochytriaceae)[17]。破囊壶菌科包含破囊壶菌属(Thraustochytrium)、日本壶菌属(Japonochytrium)、裂殖壶菌属(Schizochytrium)、吾肯氏壶菌属(Ulkenia)、橙黄壶菌属(Aurantiochytrium)、葫芦状壶菌属(Sicyoidochytrium)、帕里蒂氏壶菌属(Parietichytrium)、葡萄串状壶菌属(Botryochytrium)和单根壶菌属(Monorhizochytrium)这9个属,具有多样性高的特点[18]。破囊壶菌不同属和种的脂质生产能力和DHA产量存在很大差异。例如,从中国南方沿海水域分离出的破囊壶菌菌株中,Thraustochytrium sp. PKU#SW1的DHA产量仅为Aurantiochytrium sp. PKU#Sed1的25.00%,为Schizochytrium sp. PKU#Mn4的18.75%[19]。如图 1A所示,形态学观察表明,营养阶段的破囊壶菌细胞在显微镜下呈圆形或椭圆形,有明显的细胞壁并且细胞内含孢子,这与之前研究的观察结果一致[20]。破囊壶菌在生态学上所扮演的重要角色和生物技术应用潜力一直备受关注。在生态学方面,腐生性的生活方式以及具备分泌蛋白酶、脂肪酶、淀粉酶和纤维素酶等多种胞外酶的能力,使破囊壶菌在难降解有机质的矿化分解和海洋碳循环方面发挥着重要作用[21-22];其次,由于破囊壶菌胞内累积的PUFA是浮游生物的营养来源,并且在沿海和大洋水域中其生物量甚至超过细菌,因此破囊壶菌被认为是海洋生态系统中食物链和食物网传递的重要一环[15]。破囊壶菌能够生产多种脂肪酸,主要包括肉豆蔻酸(myristic acid, MA, C14:0)、棕榈酸(palmitic acid, PA, C16:0)等饱和脂肪酸(saturated fatty acids, SFA),以及花生四烯酸(arachidonic acid, ARA, C20:4)、二十碳五烯酸(eicosapentaenoic acid, EPA, C20:5)、二十二碳五烯酸(docosapentaenoic acid, DPA, C22:5)和DHA (C22:6)等长链PUFA[23]。如图 1B所示,用尼罗红染色后,在荧光显微镜下可观察到破囊壶菌细胞内的金黄色脂滴,并且荧光强度会随着脂质含量的增加而增强。此外,破囊壶菌能产生角鲨烯、类胡萝卜素等具备多种生物活性功能的萜类化合物,在食品、医药及化妆品等行业具有极高的应用价值[10]。在水产养殖行业,将破囊壶菌用作饲料添加剂不仅可以降低鱼类幼鱼等水产动物的发病率、提高其存活率,同时还能增加水产动物的DHA含量,从而提升水产品的质量[24]。
|
| 图 1 破囊壶菌在显微镜下的营养细胞形态(A)和尼罗红染色(B)照片 Figure 1 The photos of thraustochytrids under the microscope about (A) nutrient cell morphology and (B) Nile red staining. |
|
|
目前,Schizochytrium、Aurantiochytrium和Thraustochytrium这3个属的破囊壶菌已被广泛研究并应用于实际发酵行业中,获得了显著的成果。本文总结了部分破囊壶菌菌株在不同发酵规模下生产DHA的结果(表 1)。Schizochytrium sp. ATCC 20888在14 000 gal发酵罐中实现了高达171.50 g/L的生物量和409.00 mg/(L·h)的DHA产率[25];Schizochytrium sp. HX-308在50 L生物反应器中产生了高达103.60 g/L的脂质产量和44.30 g/L的DHA产量[26];Schizochytrium sp. SH103在5 L发酵罐中达到了2.10 g/(L·h)的生物量产率和1.00 g/(L·h)的脂质产率[27];这些数据是目前已报道的最高值。然而破囊壶菌生产DHA的产品成本(约35−40美元/kg)仍然高于传统鱼油(约16−20美元/kg),这在一定程度上限制了破囊壶菌应用于商业生产[32]。因此,降低生产成本并提高DHA产量仍然是破囊壶菌工业化大规模生产DHA需要攻克的难题。当前提升破囊壶菌DHA产量的策略主要包括分离并筛选高产菌株、优化培养参数和发酵策略、调整发酵工艺、合成生物学与代谢工程策略、诱变育种以及适应性实验室进化等[33-36]。同时,由于改变环境因子策略操作简单、成本低,破囊壶菌在环境胁迫下的响应以及通过调整环境因子以提高DHA产量的发酵工艺成为研究热点之一。
| Strains | Carbon and nitrogen source | Fermentation scale and modes | Biomass (g/L) |
Lipid yield (g/L) |
DHA yield (g/L) |
DHA productivity (mg/(L·h)) |
References |
| Schizochytrium sp. ATCC 20888 | Glucose | 14 000 gal fermenter | 171.50 | ‒ | 35.32 | 409.00 | [25] |
| Aurantiochytrium limacinum SR21 | Glucose, glycerol; yeast extract, monosodium glutamate | 5 L fermenter, fed-batch fermentation | 88.32 | 74.05 | 32.36 | 337.10 | [25] |
| Schizochytrium sp. HX-308 | Glucose; yeast extract | 50 L bioreactor, fed-batch fermentation | 151.00 | 103.60 | 44.30 | 369.10 | [26] |
| Schizochytrium sp. SH103 | Glucose; yeast extract | 5 L fermenter, fed-batch fermentation | 100.60 | 48.60 | 15.60 | 324.10 | [27] |
| Schizochytrium sp. CCTCC M209059 | Glucose; monosodium glutamate | 7 000 L fermenter, fed-batch fermentation | 90.10 | 49.16 | 19.72 | 136.90 | [28] |
| Schizochytrium sp. HX-308 | Glucose; yeast extract | 50 L bioreactor, fed-batch fermentation | 117.60 | 58.90 | 31.00 | 258.60 | [29] |
| 7 000 L bioreactor, fed-batch fermentation | 128.30 | 74.80 | 39.20 | 326.50 | |||
| Schizochytrium sp. S31 | Glycerol; yeast extract | 30 L fermenter, fed-batch fermentation | 151.40 | 79.74 | 28.93 | 301.00 | [30] |
| Schizochytrium sp. HX-308 | Glucose; monosodium glutamate | 500 L fermenter, fed-batch fermentation | 122.37 | 69.83 | 38.42 | 320.17 | [31] |
| ‒:列举的相关文献未标明具体数据 ‒: The corresponding literature cited did not specify specific data. |
|||||||
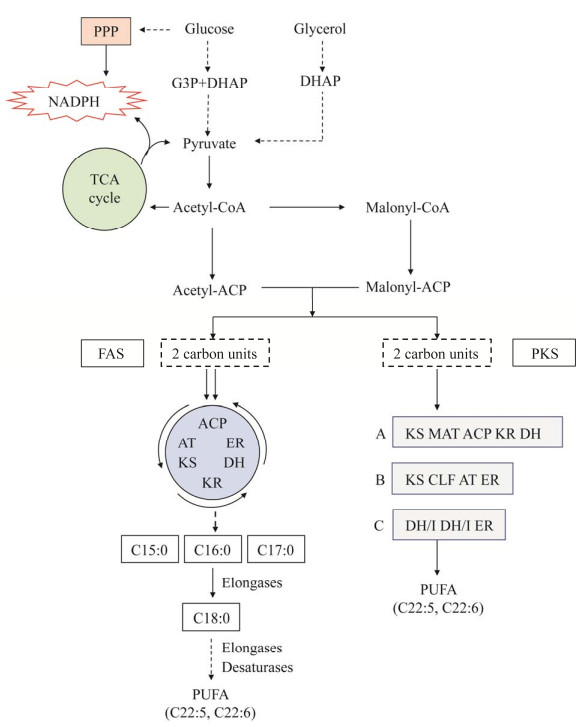
破囊壶菌有两条脂肪酸合成途径,即脂肪酸合成酶(fatty acid synthase, FAS)途径和聚酮合酶(polyketide synthase, PKS)途径,前者通过具有氧气依赖性的去饱和酶引入双键,被称为“需氧途径”,而PKS途径可在无氧条件下合成DHA,被称为“厌氧途径”[37]。两条途径均以乙酰辅酶A (acetyl-CoA)为底物、腺嘌呤核苷三磷酸(adenosine triphosphate, ATP)为能量载体和还原型烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate, NADPH)为还原力合成脂肪酸[38]。FAS途径首先由酶催化一系列迭代反应(缩合、还原、脱水和还原),每个循环增加2个碳,经过7次循环,合成饱和脂肪酸C16:0[39]。C16:0在延长酶的作用下生成C18:0,C18:0首先在△9、△12和△15去饱和酶的作用下依次生成C18:1、C18:2和C18:3,随后在各种延长酶和去饱和酶(△6、△5和△4去饱和酶)的作用下生成C22:5、C22:6等PUFA[40]。此外,FAS途径还能以丙酰辅酶A为前体合成C15:0、C17:0等奇数碳饱和脂肪酸[41]。PKS途径不涉及去饱和酶,而是直接催化DHA等PUFA的从头合成[42]。该途径是由3个亚基(亚基A、亚基B和亚基C)组成的多功能复合系统,每个亚基包含不同的结构域[β-酮酰基合酶(β-ketoacyl synthase, KS),丙二酰辅酶A: ACP酰基转移酶(malonyl-CoA: ACP transacylase, MAT),酰基载体蛋白(acyl-carrier protein, ACP),β-酮基还原酶(β-ketoreductase, KR),脱水酶(dehydratase, DH),链长因子(chain length factor, CLF),酰基转移酶(acyl transferase, AT),烯酰还原酶(enoyl-reductase, ER),脱水酶/异构酶(dehydratase/isomerase, DH/I)],由一系列酶催化底物乙酰辅酶A和丙二酰辅酶A (malonyl-CoA),经过缩合、还原、脱水、还原/异构等循环和连续反应,每个循环增加2个碳,最终合成DHA等PUFA[43]。FAS途径和PKS途径合成DHA的通路示意图见图 2。
|
| 图 2 破囊壶菌合成脂肪酸的通路示意图[39] Figure 2 Schematic diagram of fatty acid synthesis pathway in thraustochytrids[39]. KS: β-ketoacyl synthase; MAT: Malonyl-CoA: ACP transacylase; ACP: Acyl-carrier protein; KR: β-ketoreductase; DH: Dehydratase; CLF: Chain length factor; AT: Acyl transferase; ER: Enoyl-reductase; DH/I: Dehydratase/isomerase; G3P: Glyceraldehyde 3-phosphate; DHAP: Dihydroxyacetone phosphate. KS:β-酮酰基合酶;MAT:丙二酰辅酶A: ACP酰基转移酶;ACP:酰基载体蛋白;KR:β-酮基还原酶;DH:脱水酶;CLF:链长因子;AT:酰基转移酶;ER:烯酰还原酶;DH/I:脱水酶/异构酶;G3P:3-磷酸甘油醛;DHAP:磷酸二羟丙酮 |
|
|
多项研究表明,破囊壶菌细胞内的FAS途径并不完整,有的菌株缺失△12去饱和酶甚至去饱和酶完全缺失,但仍能合成DHA等PUFA[44-45],说明PKS途径在这些菌株内负责PUFA的合成。另外,FAS途径合成DHA需要消耗26个NADPH,而PKS途径只需要消耗14个NADPH并且催化步骤更少,所以通过PKS途径合成DHA的效率更高[46]。尽管当前的研究认为破囊壶菌合成DHA的两条途径是独立存在的,但是真实的DHA合成机制可能更加复杂,目前人们仅获得了初步的认识。此外,不同破囊壶菌细胞中DHA合成的两条途径是否完整存在以及两条途径之间的关系尚不明确,但当前的技术手段还无法验证这些问题,未来仍需要进一步地研究和探索。
2 不同环境胁迫对破囊壶菌DHA生产的影响 2.1 NaCl胁迫作为海洋微生物,破囊壶菌需要一定的盐度以维持其正常生长所需的渗透压,NaCl浓度变化介导的渗透压变化会显著影响破囊壶菌的生理和代谢过程[47]。虽然破囊壶菌分离自盐度不同的海洋环境并且对盐度具有良好的耐受性,但是实验室培养时一般将盐度设置在2.0%−3.4%的范围[18]。破囊壶菌在无盐或者高盐的极端盐度环境下,生长会受到极大限制甚至停止生长[48],但是菌株Schizochytrium limacinum SR21在200%海水浓度的高盐度环境中仍能较好地生长[49];Schizochytrium sp. S056和Schizochytrium mangrovei Sk-02在无盐条件下同样可以生长[50-51]。这表明破囊壶菌对盐度的耐受程度具有菌株特异性。破囊壶菌在允许其生长的盐度范围之内,生物量呈现出随盐度增加先升高后降低的总体规律,并且破囊壶菌生长的最佳盐度在不同菌株中也有所差异,这可能与菌株自身的遗传特性、分离环境以及培养条件有关[12, 50]。
关于高浓度NaCl对破囊壶菌脂质积累和脂肪酸组成影响的研究已广泛开展。人工海水浓度从25%增加到100%时,Thraustochytrium striatum ATCC 24473的C16:0、C18:0等SFA含量提高,PUFA的主要成分ARA、EPA和DHA的含量均降低[12]。Schizochytrium sp. HX-308在30 g/L高浓度NaCl条件下的脂质产量和脂质含量均低于无NaCl条件,SFA中的C14:0和C16:0含量较对照组分别增加117.58%和24.34%,PUFA中的EPA、DPA和DHA含量较对照组分别减少26.35%、17.13%和5.64%[13]。以上研究表明高盐度有利于SFA的积累而不利于PUFA的积累,这可能是为了减缓细胞膜的流动性以减少Na+的过量摄入,从而提高细胞的耐盐性[52]。然而另有研究表明高盐度会促进DHA的积累。海盐浓度从0%增加到2.00%时,Schizochytrium sp. S056在2.00%海盐浓度下有最高的DHA产量6.61 g/L[50]。海盐浓度从0 g/L增加到30 g/L时,S. mangrovei Sk-02的DHA产量从1.90 g/L提高到4.20 g/L[53]。因此,破囊壶菌在高盐度条件下的脂肪酸组成变化和DHA产量在不同菌株之间具有差异。然而目前少有研究从分子水平解析NaCl和脂肪酸合成之间的因果关系,尽管Jiang等[13]从基因表达和脂质代谢的水平阐述了破囊壶菌在盐胁迫下的调控机制,但是总体而言NaCl调节脂肪酸合成代谢通路变化的机理尚不清楚。
2.2 温度胁迫温度被认为是影响破囊壶菌生长和脂肪酸积累最重要的环境因子之一。温度胁迫下,细胞形态、细胞膜流动性、酶活性和蛋白质等生物大分子的结构和功能将会受到显著影响[54-55]。此外,低温会增加培养基中的溶解氧浓度,这可能使细胞面临氧化胁迫[56]。通常情况下,破囊壶菌可以在15−35 ℃的温度范围内生长和繁殖,其生物量随温度升高而增加,直至最佳温度时达到最高值[57-58]。表 2总结了不同破囊壶菌菌株生长的最佳温度,最佳生长温度主要分布在25−30 ℃之间,但是在不同菌株之间有所差异。
| Strains | Culture temperature (℃) |
Optimum temperature for the growth (℃) |
Maximum biomass (g/L) |
Optimum temperature for the DHA production (℃) |
Maximum DHA yield (g/L) | References |
| S. limacinum SR21 | 10−35 | 25 | − | − | − | [49] |
| S. mangrovei Sk-02 | 25−40 | 30 | 17.60 | 25 | 3.50 | [53] |
| Schizochytrium sp. HX-308 | 15−35 | 30 | 84.40 | 30 | 4.00 | [57] |
| Aurantiochytrium sp. FN21 | 15−35 | 28 | 41.41 | 28 | 7.42 | [58] |
| Aurantiochytrium sp. shy | 10−35 | 25 | 8.01 | − | − | [59] |
| Thraustochytriidae sp. Z105 | 4−35 | 25 | 16.74 | 20 | 1.32 | [60] |
| Thraustochytrium sp. FJN-10 | 15−30 | 28 | 13.60 | − | − | [61] |
| Schizochytrium sp. ZR-01 | 20−37 | 28 | 23.50 | 28 | 6.90 | [62] |
| Aurantiochytrium sp. PKU#SW7 | 15−30 | 28 | − | 28 | − | [63] |
| Thraustochytriidae sp. PKU#Mn16 | 15−30 | 28 | − | 28 | − | [63] |
| Thraustochytrium sp. ATCC 26185 | 18−33 | 28 | 6.30 | − | − | [64] |
| Schizochytrium sp. HX-308 | 15−34 | 30 | − | − | − | [65] |
| ‒:列举的相关文献未测定或标明具体数据 ‒: The corresponding literature cited did not provide measurements or specify specific data. |
||||||
升高温度可以增加C16:0等SFA的含量[66-67],降低温度可以增加DHA等PUFA的含量,但是会降低生物量和脂质产量[57, 68-69]。破囊壶菌在不同温度胁迫下的脂肪酸组成变化是为了更好地适应环境,高温胁迫下增加SFA含量能够提高细胞的耐热性,而低温胁迫下积累更多的PUFA是为了提高细胞膜的流动性,保证细胞膜在低温胁迫下的正常功能[57]。有研究尝试从基因表达的水平解释低温增加DHA等PUFA含量的机制。转录组学分析表明,低温对Schizochytrium sp. TIO01的PKS途径无影响,但是FAS途径下调[45];而低温下Aurantiochytrium sp. SD116的PUFA合成酶的基因表达量显著增加,但是I型脂肪酸合成酶的基因表达量无显著差异[70]。这说明低温胁迫下不同破囊壶菌对两条脂肪酸合成途径的调控机制存在差异,现阶段的研究尚不能阐明低温调节DHA合成的具体机制。
温度降低使DHA含量增加,这可能引起DHA产量的相同变化。例如,温度由37 ℃降低到25 ℃时,S. mangrovei Sk-02的DHA含量由26.70%增加到40.30%,DHA产量由1.60 g/L提高到3.50 g/L[53]。然而一些菌株获得最高DHA产量的温度要高于获得最高DHA含量的温度。Thraustochytriidae sp. PKU#Mn16在16 ℃条件下的DHA含量最高,但是28 ℃条件下有最高的DHA产量9.40 g/L,这是因为28 ℃条件下的生物量和TFA含量远高于16 ℃条件[71]。Schizochytrium sp. HX-308在20 ℃条件下的DHA含量最高,为51.28%,但是DHA产量在30 ℃时最高,为4.00 g/L,这是因为菌株在30 ℃下的生物量和脂质产量最高[57]。因此,DHA产量不仅与DHA含量有关,还会受到生物量、脂质产量和TFA含量的影响。在实际生产中仅考虑降低温度以提升DHA含量并不充分,还需综合考虑低温胁迫对生物量和TFA积累限制造成的DHA产量降低。
此外,当前关于温度对破囊壶菌脂肪酸组分变化的研究是针对整个细胞而言,但是细胞膜脂肪酸组分和细胞内脂肪酸组分对细胞有不同的生理意义,细胞内脂肪酸主要与能量储存有关,而细胞膜脂肪酸的组成与细胞膜流动性和抵御环境胁迫紧密相关,未来可围绕温度对细胞膜和细胞内脂肪酸组成变化的影响分别展开研究,以填补这一研究内容的空缺。
2.3 溶氧胁迫作为一种需氧微生物,破囊壶菌的生长和代谢需要一定的氧气,所以溶氧是破囊壶菌发酵过程中的关键性限制因素。随着溶氧的增加,破囊壶菌对底物的消耗速率加快,从而有利于细胞的快速生长,缩短发酵周期[72]。溶氧从10%增加到50%时,Thraustochytriidae sp. PKU#Mn16的生长速率不断加快,在50%的高溶氧条件下获得最高的生物量68.98 g/L[71]。然而持续提高溶氧并不会使生物量一直增加。转速从50 r/min提高到120 r/min时,T. striatum ATCC 24473的生物量增加4倍,但是转速进一步增加到200 r/min和300 r/min使生物量略微降低[12];Schizochytrium sp. S31在高供氧(600 r/min)条件下的生长速率和最高生物量与中等供氧(450 r/min)条件相近[73]。因此过高的溶氧反而对细胞生长不利,原因可能是高转速下的剪切应力会降低细胞活力,并且过量的溶氧可能会对细胞造成潜在的氧化损伤,从而抑制细胞生长[12]。
增加溶氧可以获得较高的脂质产量。例如,Schizochytrium sp. HX-308在400 r/min的高供氧条件下有最高的脂质产量67.31 g/L,高于正常供氧条件下的58.66 g/L[72]。但是Schizochytrium sp. HX-308在1.0 vvm的中等曝气速率下脂质产量最高,为55.30 g/L,曝气速率增加至1.5 vvm时脂质产量略有降低[74];Schizochytrium sp. HX-308在体积氧传递系数(KLa)为88.5 h‒1的中等供氧水平下可获得最高的脂质产量32.40 g/L,当KLa升高为150.1 h‒1时最终脂质产量降低为24.80 g/L[75]。因此,溶氧过高反而会降低脂质产量,这是因为过高的溶氧会使脂质过氧化造成脂质损耗,并且高溶氧下的破囊壶菌会在发酵后期分泌更多的有机酸使发酵液呈酸性,同样不利于脂质的积累[76-77]。
尽管高溶氧有助于细胞生长和脂质积累,但是降低溶氧会提高DHA等PUFA的含量[73]。溶氧由30%降至10%时,Aurantiochytrium sp. PKU#Mn16的DHA产量由4.70 g/L增加到6.00 g/L[78];Aurantiochytrium sp. T66的DHA含量在限氧条件下提高到52%[79]。如前所述,FAS途径中的去饱和酶依赖于氧气,所以相较于PKS途径,FAS途径中的去饱和酶活性更容易受到溶氧浓度的影响[80]。关于Aurantiochytrium sp. PKU#Mn16的转录组学分析表明,进入FAS途径被继续合成为DHA的SFA会在低氧供应下减少通量[78]。因此,低溶氧条件下通过FAS途径合成的不饱和脂肪酸减少,而PKS途径的非氧气依赖性可能使其比FAS途径更活跃,从而引导更多碳源进入PKS途径合成DHA,最终DHA等PUFA的相对含量增加[74]。
2.4 pH胁迫pH是破囊壶菌发酵过程中重要的参数,从多方面影响细胞的生长和代谢。培养基的pH会改变细胞膜的电荷和通透性,还会干扰酶活性,从而影响物质的吸收与运输和细胞的代谢活动[81-82]。在破囊壶菌的发酵过程中,随着细胞密度的增加,培养基中的底物不断被消耗,同时细胞会分泌一些代谢物到发酵液中,使培养基的pH发生变化,例如,培养基中的硫酸铵被消耗以及糖代谢分泌的有机酸会降低发酵液的pH[74, 83];而含氨基氮源的代谢可能导致培养基碱化[84]。多项研究通过实时监测证实破囊壶菌发酵过程中发酵液的pH不断变化,最终发酵液的pH会高于起始pH[26, 84-85]。因此,发酵液的pH改变与发酵阶段、培养基组分、培养条件等多种因素相关。
表 3总结了破囊壶菌生长的最佳pH,大多数破囊壶菌生长的最佳pH值分布在6.0−7.0之间,酸性pH值(< 5.0)以及碱性pH值(> 8.0)会显著抑制破囊壶菌生长[91]。这可能是因为接近中性pH环境下的酶活性更高,有利于底物的消耗和细胞生长;而酸性pH会影响细胞膜的通透性和酶活性,碱性pH会降低与酶活性相关金属离子的溶解性而干扰酶活性,从而不利于细胞生长[85]。将pH值从中性降低到5.0或6.0可以提高破囊壶菌的TFA含量[12, 53]。然而,以醋酸为碳源培养S. limacinum SR21,pH值为7.0时TFA含量最高,将pH值降低到6.0使TFA产量降低40.00%[86],这说明脂肪酸积累的最适pH值会受到培养基组分的影响。将pH值从7.0降低到4.0,Schizochytrium sp. AB-610的脂质含量由37.88%提高到42.01%;该菌株在pH值为7.0时有最高生物量62.63 g/L,pH值为5.0时生物量略有降低,pH值为4.0时生物量急剧降低,这使得脂质和DHA产量也随之降低[85]。因此,尽管酸性条件会提高脂质和DHA含量,但是由于对细胞生长不利,所以最终会降低脂质和DHA的产量。如表 3所示,破囊壶菌在pH值为6.0−7.0能够获得最高的DHA产量,而中性和弱酸性的pH同样最适合脂质生产。Schizochytrium sp. HX-308和S. limacinum SR21在pH值为7.0时有最高脂质产量,分别为51.76 g/L和7.76 g/L[87-88],Thraustochytriidae sp. Z105在pH值为6.0时有最高脂质产量4.07 g/L[60]。然而目前少有研究针对pH调控破囊壶菌DHA生产的分子机理展开,所以酸性环境促进脂质积累和DHA合成的分子机制尚未可知,未来应借助组学工具填补这一研究内容的缺乏,从而为提高破囊壶菌DHA生产的pH调控提供更多理论依据。
| Strains | Culture pH | Optimum pH for the growth | Maximum biomass (g/L) | Optimum pH for the DHA production | Maximum DHA yield (g/L) | References |
| Aurantiochytrium limacinum PKU#SW8 | 3.0−9.0 | 4.0 | 6.49 | 4.0 | 1.00 | [33] |
| S. mangrovei Sk-02 | 4.0−7.0 | 6.0 | 18.30 | 6.0 | 3.90 | [53] |
| Aurantiochytrium sp. shy | 6.0−8.0 | 6.0 | 6.77 | ‒ | ‒ | [59] |
| Thraustochytriidae sp. Z105 | 3.0−9.0 | 6.0 | 14.55 | 6.0 | 1.22 | [60] |
| Aurantiochytrium sp. PKU#SW7 | 4.0−8.0 | 6.0 | 6.96 | 6.0 | 1.40 | [63] |
| Thraustochytriidae sp. PKU#Mn16 | 4.0−8.0 | 6.0 | 6.79 | 6.0 | 1.43 | [63] |
| Schizochytrium sp. S31 | 5.0−8.0 | 7.0 | 6.01 | 7.0 | 0.31 | [83] |
| Schizochytrium sp. AB-610 | 4.0−8.0 | 7.0 | 62.63 | ‒ | ‒ | [85] |
| S. limacinum SR21 | 6.0−7.0 | 7.0 | 139.10 | 7.0 | 20.45 | [86] |
| Schizochytrium sp. HX-308 | 4.0−8.0 | 7.0 | 97.11 | 6.5 | 23.36 | [87] |
| S. limacinum SR21 | 6.0−9.0 | 7.0 | 44.90 | 7.0 | 0.58 | [88] |
| Aurantiochytrium sp. FIKU018 | 6.0−7.0 | 6.0 | 13.93 | ‒ | ‒ | [89] |
| Aurantiochytrium sp. SA-96 | 4.0−8.0 | 7.4 | 12.40 | ‒ | ‒ | [90] |
| ‒:列举的相关文献未测定或标明具体数据 ‒: The corresponding literature cited did not provide measurements or specify specific data. |
||||||
第2章节总结了NaCl、温度、溶氧和pH这4种环境胁迫因子对破囊壶菌生长、脂质积累和DHA生产的影响,接下来的第3章节将从渗透调节、分段发酵和缓解氧化应激3个方面综述基于环境胁迫的发酵策略在提高DHA产量方面的研究现状(图 3)。

|
| 图 3 环境因子对破囊壶菌生长和脂质积累的影响以及基于环境胁迫的发酵策略[46] Figure 3 Effects of environmental factors on the growth and lipid accumulation of thraustochytrids and fermentation strategies based on environmental stresses[46]. DCW: Dry cell weight; ROS: Reactive oxygen species; T: Temperature; DO: Dissolved oxygen. DCW:细胞干重;ROS:活性氧;T:温度;DO:溶氧 |
|
|
高浓度NaCl对发酵罐有强烈的腐蚀作用,这不利于发酵设备的长期使用和维护,而使用更耐腐蚀的钢材料会增加发酵设备的成本[92]。因此,筛选低浓度NaCl条件下仍然具备良好生长和生产能力的菌株对于降低生产成本具有重要意义。Thraustochytrium sp. ATCC 26185和Aurantiochytrium sp. TWZ-97均能在低浓度NaCl条件下生长良好并且积累更多的角鲨烯,两者分别在5 g/L NaCl和7.5 g/L NaCl浓度下有最高的角鲨烯产量123.60 mg/L和140.40 mg/L[93-94]。这表明某些破囊壶菌菌株可以实现低浓度NaCl条件下的发酵生产。菌株ACEM C、ACEM A和ACEM E在低浓度NaCl条件下培养时,用甘露醇或蔗糖将渗透压调整为与500 mmol/L NaCl相当的渗透压,细胞表现出正常的生长模式甚至获得比对照组更高的生物量[47, 95]。在含有10 mmol/L低NaCl浓度的培养基中添加蔗糖和甘露醇并调整渗透压至最佳值,可以使菌株ACEM A的PUFA含量由33.00%分别增加到45.00%和48.00%[95]。这表明降低NaCl浓度并添加适量有机渗透剂的渗透调节策略具有可行性。此外,非氯钠盐替代策略或许也是一种可行的方法。用1%的硫酸钠代替2%的海盐培养Schizochytrium sp. S056,培养基的渗透压由1 346 mOsm/L降至857 mOsm/L,但是细胞干重从34.76 g/L提高到38.93 g/L,DHA产量从6.61 g/L提高到7.95 g/L[50]。综上所述,低浓度NaCl胁迫下破囊壶菌的不同培养方式为不同菌株在大规模发酵生产中的应用提供了可行的依据,但是仍需要中试放大规模的试验进一步验证。
3.2 分段发酵策略虽然环境胁迫能够诱导脂质积累,但是往往以生物量的减少为代价,由于脂质和DHA产量受到生物量的影响,因此在破囊壶菌的整个发酵过程中一直施加环境压力并不能获得高生物量和高脂质产量。破囊壶菌的发酵过程可以分为2个阶段:第1阶段是细胞的快速生长阶段,该时期细胞数量迅速增加,碳氮源等营养物质消耗速率加快,对氧气的需求量较高;第2阶段是脂质积累阶段,当氮源耗尽后细胞开始大量合成脂质,对氧气的消耗减少[75]。因此,根据破囊壶菌的发酵特点适时施加一定环境压力的两阶段或三阶段发酵策略或许可以改善脂质生产的限制,即在细胞的生长阶段提供充足的营养和适宜的环境条件以获得较高生物量,在脂质积累阶段施加一定的环境压力以促进DHA和脂质合成。表 4对现阶段利用温度、溶氧和pH的分阶段培养策略在提高破囊壶菌DHA生产中的研究进行了总结。
| Strains | Staged fermentation strategies | Fermentation scale and modes | Biomass (g/L) |
DHA yield (g/L) |
References |
| Schizochytrium sp. HX-308 | The aeration rate was set to 1.0 vvm for 48 h then changed to 0.5 vvm for 72 h | 50 L bioreactor, fed-batch fermentation | 151.00 | 44.30 | [26] |
| Schizochytrium sp. HX-308 | The temperature was set to 30 ℃ for 32 h then changed to 20 ℃ for 12 h | 250 mL shaking flask, batch fermentation | ‒ | 51.98% TFA 6.05% DCW |
[57] |
| S. limacinum SR21 | Dissolved oxygen was controlled at 50% in a 5 L fermenter for 40 h then transferred to 250 mL shaking flasks for 200 h | 5 L fermenter and 250 mL shaking flask, batch fermentation | 37.90 | 6.56 | [77] |
| S. limacinum SR21 | The aeration rate was set to 1.0 vvm in the growth stage then changed to 0.5 vvm in the lipogenic stage | 5 L bioreactor, fed-batch fermentation | 122.00 | 15.25 | [86] |
| The aeration rate was set to 1.5 vvm in the growth stage then changed to 1.0 vvm in the lipogenic stage | 141.40 | 20.64 | |||
| Schizochytrium sp. HX-308 | HCl and NaOH were used as pH regulators, the pH was set to 7.0 for 36 h then changed to 5.0 for 84 h | 5 L bioreactor, fed-batch fermentation | 98.07 | 25.85 | [87] |
| Citric acid and NaOH were used as pH regulators, the pH was set to 7.0 for 36 h then changed to 5.0 for 84 h | 106.44 | 30.80 | |||
| Schizochytrium sp. ATCC 20888 | The temperature was set to 26 ℃ for 48 h then changed to 22 ℃ for 96 h | 50 L fermenter, batch fermentation | ‒ | 6.50 | [96] |
| Schizochytrium LX0809 | The agitation speed was kept at 400 r/min for 40 h then shifted to 300 r/min for 56 h | 10 L fermenter, fed-batch fermentation | 92.00 | 21.00 | [97] |
| Schizochytrium sp. CCTCC M209059 | The aeration rate was controlled at 0.4 vvm for 24 h, then shifted to 0.6 vvm until 96 h, and then switched back to 0.4 vvm until the end of the fermentation | 1500 L fermenter, fed-batch fermentation | 71.00 | 15.76 | [98] |
| Schizochytrium sp. FJU-512 | Dissolved oxygen was controlled above 20% for 48 h then reduced to 5% for 72 h, the temperature was set to 28 ℃ for 96 h then to 25 ℃ for 24 h | 15 L fermenter, fed-batch fermentation | 103.90 | 16.00 | [99] |
| ‒:列举的相关文献未测定或标明具体数据 ‒: The corresponding literature cited did not provide measurements or specify specific data. |
|||||
高温可以加速细胞生长,而低温可以提高DHA含量,所以高温到低温的分阶段变温调控策略或许可以弥补恒温培养所带来的生物量或DHA积累的限制。在摇瓶中培养Aurantiochytrium sp. FN21,采用28 ℃ (0−84 h)、25 ℃ (84−108 h)和15 ℃ (108−120 h)的三阶段控温策略,最终获得的生物量与28 ℃恒温培养相近,DHA含量从40.94%提高到46.39%,DHA产量从7.42 g/L提高到8.29 g/L[58]。用250 mL摇瓶培养Schizochytrium sp. HX-308,采用30 ℃培养32 h和20 ℃培养12 h的两阶段控温策略,获得的DHA含量为51.98% (TFA)和6.05% (DCW);但是在30 ℃的恒温条件下,该菌株的DHA含量在培养30 h后达到52.02% (TFA),培养42 h后达到5.82% (DCW)[57],可以看出该研究中的变温策略并无显著优势。此外,低温控制会增加生产成本,因此工业生产DHA可能无须应用变温控制策略。
根据破囊壶菌在发酵过程中对氧气需求量变化的特点,以及高溶氧促进生长、低溶氧提高DHA含量的规律,研究人员对高溶氧到低溶氧分段式发酵策略的效果进行了探究。在50 L发酵罐中培养Schizochytrium sp. HX-308,采用KLa控制在150.1·h‒1 (0−40 h)和88.5·h‒1 (40−160 h)的两阶段控氧策略,生物量和DHA产量分别达到92.72 g/L和17.70 g/L,比KLa为88.5·h‒1的恒溶氧条件分别提高了26.84%和63.89%[75]。尽管当前进行了一些分段供氧策略的研究,并且在提高生物量和DHA产量方面有较好的效果,但是这些研究多集中于实验室小试和中试规模,对于大规模培养的可行性不能确定。尤其是大型生物反应器的分批培养容易出现低氧状态,除了发酵的初始阶段,一直保持发酵液的高溶氧浓度难以实现[100]。因此,分阶段供氧策略的必要性和可行性还有待验证。
接近中性的pH有利于破囊壶菌生长,降低pH能够提高脂质和DHA含量,利用这一特点在发酵过程中适时调节pH同样有利于提高脂质和DHA产量。以8 mol/L的HNO3和NaOH溶液调节培养液的pH,pH值控制在7.0培养60 h然后将pH值降低到5.0继续培养60 h的两段式发酵策略,使Schizochytrium sp. AB-610的脂质产量达到26.00 g/L,DHA产量达到11.44 g/L,分别比无pH控制策略(21.85和9.36 g/L)提高了19.00%和22.20%[85]。虽然pH的分段调控策略对于提高破囊壶菌的DHA产量有一定效果,但是许多研究在大规模发酵过程中并未进行pH调控或者将pH控制在近中性条件下,DHA生产同样获得了较好的结果[29-30]。此外,pH调节剂的选择以及添加pH调节剂可能带来的成本问题也应该在考虑的范围之内,所以尚不能确定实行该策略的必要性。
总而言之,基于温度、溶氧和pH的分阶段发酵策略可以为后续破囊壶菌的DHA工业化生产和实际应用提供借鉴,但是这些策略在提高DHA产量方面的效果不一,并且存在一些问题。一方面,改变这些环境因子条件的精确时间难以确定,从而增加了实际操作的难度;另一方面,调控这些环境因子可能会增加生产成本。同时,很多大规模发酵在最佳的恒定条件下也能够获得较高的DHA产量,因此当前分阶段发酵在提高DHA产量方面的优势并不突出,该策略是否能够在大规模发酵中有效运行并且取得显著的效果还有待于进一步研究。
3.3 缓解氧化应激提高DHA生产的策略细胞在有氧条件下会不可避免地产生活性氧(reactive oxygen species, ROS),在稳定条件下,ROS的产生与清除处于平衡状态,这依赖于细胞自身的抗氧化防御系统发挥作用[72]。然而环境胁迫会诱导ROS过量积累,从而造成细胞的氧化应激[101]。ROS不仅使胞内脂质过氧化从而降低PUFA和脂质的产量,而且会对DNA、蛋白质以及细胞膜造成氧化损伤,影响细胞膜的正常功能,甚至造成细胞死亡[102]。研究表明,破囊壶菌在不同环境胁迫下会提高抗氧化酶基因的表达水平来增加抗氧化酶的含量,从而提高细胞的抗氧化能力以抵御氧化应激(表 5)。在工业大规模发酵过程中,仅依靠细胞自身的抗氧化能力不足以缓解氧化应激造成的脂质过氧化,这使得脂质和DHA产量存在一定程度的损失,但是该过程往往被忽视。因此人们尝试了多种提高细胞抗氧化能力的策略以缓解脂质过氧化,从而提高脂质和DHA的产量。
| Strains | Environmental stresses | Control condition | Antioxidant enzymes | Differential gene expression levels compared with the control | References |
| Schizochytrium sp. HX-308 | High salinity of 30 g/L NaCl | 0 g/L NaCl | SOD | Increased by 3.30 times | [13] |
| APX | Two genes increased by 2.31 and 1.84 times | ||||
| Schizochytrium sp. FJU-512 | Low temperature of 15 ℃ | 28 ℃ | SOD | Increased by 8.84 times | [56] |
| Schizochytrium sp. HX-308 | High oxygen supply of 400 r/min | 300 r/min | CAT | Increased by 1.54 times | [72] |
| SOD:超氧化物歧化酶;CAT:过氧化氢酶;APX:抗坏血酸过氧化物酶 SOD: Superoxide dismutase; CAT: Catalase; APX: Ascorbate peroxidase. |
|||||
适应性实验室进化(adaptive laboratory evolution, ALE)是对菌株施加特定或者逐渐增加的环境压力,在细胞的连续传代培养中对细胞进行驯化,最终得到具备优良性状的进化菌株[103]。ALE能够在驯化的过程中逐渐提高细胞对环境胁迫的耐受力和适应性,是改善细胞生长和代谢表型的有效途径,逐渐成为发酵行业微生物育种的有力工具[104]。
目前,一些研究运用ALE选育耐胁迫的破囊壶菌菌株,旨在获得同时具备高生物量和高脂质产量的进化菌株,利用高盐度、高温、低温和高氧的ALE均已开展。基于高盐度(30 g/L NaCl)对菌株Schizochytrium sp. HX-308进行150 d的驯化后得到进化菌株ALE150,ALE150的细胞干重和脂质产量分别达到134.50 g/L和80.14 g/L,比初始菌株提高32.70%和53.31%,同时检测到ALE150有更高的总抗氧化能力(total antioxidant capacity, T-AOC)和更低的ROS水平[52]。应用34.5 ℃的高温驯化菌株Schizochytrium sp. CCTCC M209059,经过70 d驯化得到进化菌株ALE70,ALE70在34 ℃条件下培养的最高细胞干重和脂质产量分别达到74.30 g/L和31.00 g/L,比起始菌株提高78.39%和148.00%;同时ROS水平和热休克蛋白的表达水平降低,菌株的耐高温能力显著增强[105]。以230 r/min的高氧为选择压力驯化Schizochytrium sp. HX-308,经过40个循环得到进化菌株ALE40,其细胞干重和DHA产量分别达到84.34 g/L和26.40 g/L,比起始菌株提高19.10%和34.83%[106]。除了单一胁迫因子的ALE,有研究利用多种胁迫因子的协同作用对破囊壶菌菌株进行驯化。例如,对Schizochytrium sp. HX-308采用4 ℃低温和30 g/L NaCl高盐度的协同双因子ALE策略,利用低温条件提高DHA含量、高盐度条件增强抗氧化能力,经过30个周期(90 d)得到的进化菌株ALE-TF30,其最大细胞干重和DHA产量分别为126.40 g/L和38.12 g/L,分别比初始菌株提高27.42%和57.52%[101]。
综上所述,ALE得到的进化菌株可以从整体水平上提高破囊壶菌对氧化应激的耐受性,该方法不仅能够显著增强细胞的抗氧化能力从而减少细胞的氧化损伤以提高生物量积累,同时能够缩短发酵周期并且有效提高破囊壶菌的脂质和DHA生产能力,从而为DHA大规模生产提供了潜力菌株和可行的育种方法。
3.3.2 外源添加化学调节剂化学调节剂可以特异性调节靶蛋白的功能和代谢途径,其中,抗氧化剂能够减缓氧化应激,在促进细胞生长的同时减少脂质过氧化造成的脂质损失[107]。近年来已有一些研究通过添加抗氧化剂的方法来改善细胞生长和脂质生产。在添加9 g/L抗坏血酸后,Schizochytrium sp. HX-308的最大细胞干重、脂质产量和DHA产量分别为106.72、65.50和34.59 g/L,比对照组提高16.16%、14.46%和30.44%,同时检测到细胞具有更高的T-AOC和更低的ROS水平[107]。在Schizochytrium sp. H016的培养基中补充外源性1 mmol/L的芝麻酚,细胞的最高脂质和DHA产量分别达到17.24 g/L和7.56 g/L,比对照组提高53.52%和78.30%,并且芝麻酚的加入增强了细胞的T-AOC,降低了ROS水平[108]。抗氧化剂没食子酸丙酯和丁基羟基甲苯均可以提高Schizochytrium sp. S31的生物量和脂质水平[109];在Schizochytrium sp. SP1的培养基中添加亚麻籽油获得了更高的脂质产量[110]。这些研究都证明外源添加抗氧化剂可以显著提高细胞的抗氧化能力,通过调节细胞内的ROS水平有效减缓发酵过程中的脂质过氧化,从而提高脂质和DHA产量,但是具体的作用机制尚不清楚。另外,有研究发现植物激素通过调节脂质代谢的途径也可提高DHA产量[111]。
3.3.3 代谢工程除了外源添加抗氧化剂,过表达抗氧化酶基因也能提高细胞的抗氧化能力。在Schizochytrium sp. PKU#Mn4中过表达超氧化物歧化酶(SOD1)基因得到重组菌株(R7),与野生型菌株相比,R7的SOD1活性提高5.20%−71.60%,ROS下降7.80%−38.50%,PUFA产量(1.23 g/L)提高32.90%,并且R7的T-AOC始终高于野生型菌株[112]。在Schizochytrium sp. ATCC 20888中过表达6种ROS清除酶和醛类清除酶基因,构建出多种工程菌株以增强细胞对氧化应激的防御能力,其中菌株OaldH-gpo-trxR最高的细胞干重、脂质和DHA产量分别为50.50、33.10和13.30 g/L,比原菌株增加18.50%、80.90%和114.50%[36]。因此,过表达抗氧化酶基因的代谢工程策略能够提高细胞的抗氧化能力和DHA产量,尽管这方面的研究相对较少,但现有的研究结果证明了此策略的可行性及有效性。此外,对DHA合成路径中以及所需前体物质代谢途径中的关键基因进行调控的代谢工程策略,包括PUFA生物合成路径中的关键酶基因以及FAS路径中的延伸酶和去饱和酶基因,也是提高DHA产量的可行策略[46]。
4 总结与展望DHA由于具备多种重要的生理功能,近年来的应用和消费市场不断扩大,破囊壶菌作为一种可再生的产油微生物,成为DHA商业生产的潜在替代来源。然而相对更高的生产成本以及较低的DHA生产率限制了破囊壶菌在工业生产中的广泛应用。因此,国内外研究者围绕降低生产成本、提高DHA产量这2个关键问题开展了大量研究,同时还应进行破囊壶菌生产DHA产品的生命周期评价以了解破囊壶菌替代鱼油的环境可持续性,从而为破囊壶菌工业化生产提供有效指导。
低温、低溶氧及酸性pH等环境胁迫会诱导破囊壶菌脂质积累,同时促进DHA等PUFA的合成,但这通常会抑制细胞的生长,还可能造成细胞的氧化损伤,从而降低DHA产量。根据破囊壶菌的发酵阶段特点和响应环境胁迫的规律开发出多种分段式培养策略,尽管这些策略在提高DHA产量方面能够取得良好的效果,但是考虑到实际操作的难度以及可能的成本问题,实行该策略的可行性和必要性还存在争议。有研究还综述了碳氮源、微量元素等营养限制对破囊壶菌生产DHA的影响以及相应的发酵工艺[100, 113]。此外,发酵过程中氧化应激造成的PUFA损耗问题应该引起重视,提高细胞的抗氧化能力是减缓氧化损伤、增加PUFA积累的关键,当前的有效方法包括外源添加抗氧化剂、过表达抗氧化酶基因以及利用环境压力的ALE。其中,添加抗氧化剂操作简单且经济有效,过表达抗氧化酶基因构建的重组菌株和ALE获得的进化菌株都具备较强的抗氧化能力这一优良性状,在提高DHA产量的规模化培养中有很大的发展潜力。
然而,目前破囊壶菌在环境胁迫下的生理代谢响应机制以及利用ALE和代谢工程的菌株改良策略仍处于初步探索阶段,尤其是环境因子调控DHA合成的分子机理不清晰,ALE进化机理不明确,脂肪酸合成路径中相关基因的功能注释和代谢通量分析不完整。未来应结合各种组学技术进行深入研究的方向有:(1) 环境因子调控破囊壶菌DHA生物合成的分子机制,为进一步推动破囊壶菌高效DHA生产工艺的开发提供理论依据;(2) 菌株在ALE进化过程中关键代谢途径和调控基因的变化,从而明确进化机制;(3) FAS和PKS路径中基因的完整功能和代谢通量,尤其是对PKS路径进行深度挖掘,从而为代谢工程提供关键的靶向基因。另外,当前对破囊壶菌DHA生产的工艺开发多集中于实验室规模,对于放大到中试规模和工业规模DHA生产效果的研究仍然较少,确定工艺放大的关键因素尤为重要,未来还要继续探索,最终为破囊壶菌应用于大规模生产DHA提供理论指导和方法依据。
| [1] |
ROLLIN S, GUPTA A, PURI M. Optimising pineapple filtrate assisted cell disruption of wet thraustochytrid biomass for improved lipid extraction[J]. Journal of Cleaner Production, 2022, 378: 134393. DOI:10.1016/j.jclepro.2022.134393 |
| [2] |
WATANABE T, SAKIYAMA R, IIMI Y, SEKINE S, ABE E, NOMURA KH, NOMURA K, ISHIBASHI Y, OKINO N, HAYASHI M, ITO M. Regulation of TG accumulation and lipid droplet morphology by the novel TLDP1 in Aurantiochytrium limacinum F26-B[J]. Journal of Lipid Research, 2017, 58(12): 2334-2347. DOI:10.1194/jlr.M079897 |
| [3] |
SUI N, WANG Y, LIU SS, YANG Z, WANG F, WAN SB. Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut[J]. Frontiers in Plant Science, 2018, 9: 7. DOI:10.3389/fpls.2018.00007 |
| [4] |
CHAUHAN AS, PATEL AK, CHEN CW, CHANG JS, MICHAUD P, DONG CD, SINGHANIA RR. Enhanced production of high-value polyunsaturated fatty acids (PUFAs) from potential thraustochytrid Aurantiochytrium sp.[J]. Bioresource Technology, 2023, 370: 128536. DOI:10.1016/j.biortech.2022.128536 |
| [5] |
KALIDASAN K, VINITHKUMAR NV, PETER DM, DHARANI G, DUFOSSÉ L. Thraustochytrids of mangrove habitats from Andaman Islands: species diversity, PUFA profiles and biotechnological potential[J]. Marine Drugs, 2021, 19(10): 571. DOI:10.3390/md19100571 |
| [6] |
BIE NN, HAN LR, MENG M, YAN ZL, WANG CL. The immunomodulatory effect of docosahexaenoic acid (DHA) on the RAW264.7 cells by modification of the membrane structure and function[J]. Food & Function, 2020, 11(3): 2603-2616. |
| [7] |
GHASEMI FARD S, WANG FL, SINCLAIR AJ, ELLIOTT G, TURCHINI GM. How does high DHA fish oil affect health? A systematic review of evidence[J]. Critical Reviews in Food Science and Nutrition, 2019, 59(11): 1684-1727. DOI:10.1080/10408398.2018.1425978 |
| [8] |
HUANG ST, LI HX, YU Y, YUAN B, CAO CJ, CHENG SJ. Research progress on physiological functions of DHA algal oil and its synergistic application in food[J]. Science and Technology of Food Industry, 2023, 44(6): 468-476. (in Chinese) 黄淑婷, 李宏新, 于越, 袁彪, 曹崇江, 程抒劼. DHA藻油的生理功能及在食品中复配协同应用的研究进展[J]. 食品工业科技, 2023, 44(6): 468-476. DOI:10.13386/j.issn1002-0306.2022050329 |
| [9] |
OLIVER L, DIETRICH T, MARAÑÓN I, VILLARÁN MC, BARRIO RJ. Producing omega-3 polyunsaturated fatty acids: a review of sustainable sources and future trends for the EPA and DHA market[J]. Resources, 2020, 9(12): 148. DOI:10.3390/resources9120148 |
| [10] |
GUPTA A, BARROW CJ, PURI M. Multiproduct biorefinery from marine thraustochytrids towards a circular bioeconomy[J]. Trends in Biotechnology, 2022, 40(4): 448-462. DOI:10.1016/j.tibtech.2021.09.003 |
| [11] |
WANG QZ, YE HK, SEN B, XIE YX, HE YD, PARK S, WANG GY. Improved production of docosahexaenoic acid in batch fermentation by newly-isolated strains of Schizochytrium sp. and Thraustochytriidae sp. through bioprocess optimization[J]. Synthetic and Systems Biotechnology, 2018, 3(2): 121-129. DOI:10.1016/j.synbio.2018.04.001 |
| [12] |
XIAO R, LI X, ZHENG Y. Comprehensive study of cultivation conditions and methods on lipid accumulation of a marine protist, Thraustochytrium striatum[J]. Protist, 2018, 169(4): 451-465. DOI:10.1016/j.protis.2018.05.005 |
| [13] |
JIANG JY, ZHU SY, ZHANG YT, SUN XM, HU XC, HUANG H, REN LJ. Integration of lipidomic and transcriptomic profiles reveals novel genes and regulatory mechanisms of Schizochytrium sp. in response to salt stress[J]. Bioresource Technology, 2019, 294: 122231. DOI:10.1016/j.biortech.2019.122231 |
| [14] |
BAGUL VP, ANNAPURE US. Isolation of fast-growing thraustochytrids and seasonal variation on the fatty acid composition of thraustochytrids from mangrove regions of Navi Mumbai, India[J]. Journal of Environmental Management, 2021, 290: 112597. DOI:10.1016/j.jenvman.2021.112597 |
| [15] |
BAI MH, SEN B, WANG QZ, XIE YX, HE YD, WANG GY. Molecular detection and spatiotemporal characterization of labyrinthulomycete protist diversity in the coastal waters along the Pearl River Delta[J]. Microbial Ecology, 2019, 77(2): 394-405. DOI:10.1007/s00248-018-1235-8 |
| [16] |
LYU L, WANG QZ, WANG GY. Cultivation and diversity analysis of novel marine thraustochytrids[J]. Marine Life Science & Technology, 2021, 3(2): 263-275. |
| [17] |
RAGHUKUMAR S. Ecology of the marine protists, the labyrinthulomycetes (thraustochytrids and labyrinthulids)[J]. European Journal of Protistology, 2002, 38(2): 127-145. DOI:10.1078/0932-4739-00832 |
| [18] |
MARCHAN LF, CHANG KJL, NICHOLS PD, MITCHELL WJ, POLGLASE JL, GUTIERREZ T. Taxonomy, ecology and biotechnological applications of thraustochytrids: a review[J]. Biotechnology Advances, 2018, 36(1): 26-46. DOI:10.1016/j.biotechadv.2017.09.003 |
| [19] |
LIU Y, SINGH P, SUN Y, LUAN SJ, WANG GY. Culturable diversity and biochemical features of thraustochytrids from coastal waters of Southern China[J]. Applied Microbiology and Biotechnology, 2014, 98(7): 3241-3255. DOI:10.1007/s00253-013-5391-y |
| [20] |
LI JJ, LIU Y, CHENG JY, MAURYCY D. Growth features and fatty acid analysis of six thraustochytrid strains from Shenzhen coastal seawater[J]. Microbiology China, 2015, 42(1): 17-23. (in Chinese) 李晶晶, 刘瑛, 成家杨, Maurycy Daroch. 深圳海域6株破囊壶菌的生长特性及油脂成分分析[J]. 微生物学通报, 2015, 42(1): 17-23. DOI:10.13344/j.microbiol.china.140415 |
| [21] |
SIRIRAK K, SUANJIT S, POWTONGSOOK S, JARITKHUAN S. Characterization and PUFA production of Aurantiochytrium limacinum BUCHAXM 122 isolated from fallen mangrove leaves[J]. ScienceAsia, 2020, 46(4): 403-411. DOI:10.2306/scienceasia1513-1874.2020.051 |
| [22] |
JUNTILA DJ, YONEDA K, SUZUKI I. Genetic modification of the thraustochytrid Aurantiochytrium sp. 18W-13a for cellobiose utilization by secretory expression of β-glucosidase from Aspergillus aculeatus[J]. Algal Research, 2019, 40: 101503. DOI:10.1016/j.algal.2019.101503 |
| [23] |
SUN XM, XU YS, HUANG H. Thraustochytrid cell factories for producing lipid compounds[J]. Trends in Biotechnology, 2021, 39(7): 648-650. DOI:10.1016/j.tibtech.2020.10.008 |
| [24] |
SOUDANT P, VENTURA M, CHAUCHAT L, GUERREIRO M, MATHIEU-RESUGE M, Le GRAND F, SIMON V, COLLET S, ZAMBONINO-INFANTE JL, Le GOÏC N, LAMBERT C, FERNANDES F, SILKINA A, de SOUZA MF, de la BROISE D. Evaluation of Aurantiochytrium mangrovei biomass grown on digestate as a sustainable feed ingredient of sea bass, Dicentrarchus labrax, juveniles and larvae[J]. Sustainability, 2022, 14(21): 14573. DOI:10.3390/su142114573 |
| [25] |
LI J, LIU RJ, CHANG GF, LI XY, CHANG M, LIU YF, JIN QZ, WANG XG. A strategy for the highly efficient production of docosahexaenoic acid by Aurantiochytrium limacinum SR21 using glucose and glycerol as the mixed carbon sources[J]. Bioresource Technology, 2015, 177: 51-57. DOI:10.1016/j.biortech.2014.11.046 |
| [26] |
GUO DS, JI XJ, REN LJ, LI GL, HUANG H. Improving docosahexaenoic acid production by Schizochytrium sp. using a newly designed high-oxygen-supply bioreactor[J]. AIChE Journal, 2017, 63(10): 4278-4286. DOI:10.1002/aic.15783 |
| [27] |
JU JH, OH BR, KO DJ, HEO SY, LEE JJ, KIM YM, YANG K, SEO JW, HONG WK, KIM CH. Boosting productivity of heterotrophic microalgae by efficient control of the oxygen transfer coefficient using a microbubble sparger[J]. Algal Research, 2019, 41: 101474. DOI:10.1016/j.algal.2019.101474 |
| [28] |
QU L, REN LJ, HUNG H. Scale-up of docosahexaenoic acid production in fed-batch fermentation by Schizochytrium sp. based on volumetric oxygen-transfer coefficient[J]. Biochemical Engineering Journal, 2013, 77: 82-87. DOI:10.1016/j.bej.2013.05.011 |
| [29] |
GUO DS, JI XJ, REN LJ, LI GL, SUN XM, CHEN KQ, GAO S, HUANG H. Development of a scale-up strategy for fermentative production of docosahexaenoic acid by Schizochytrium sp.[J]. Chemical Engineering Science, 2018, 176: 600-608. DOI:10.1016/j.ces.2017.11.021 |
| [30] |
CHANG GF, GAO NS, TIAN GW, WU QH, CHANG M, WANG XG. Improvement of docosahexaenoic acid production on glycerol by Schizochytrium sp. S31 with constantly high oxygen transfer coefficient[J]. Bioresource Technology, 2013, 142: 400-406. DOI:10.1016/j.biortech.2013.04.107 |
| [31] |
GUO DS, TONG LL, JI XJ, REN LJ, DING QQ. Development of a strategy to improve the stability of culture environment for docosahexaenoic acid fermentation by Schizochytrium sp.[J]. Applied Biochemistry and Biotechnology, 2020, 192(3): 881-894. DOI:10.1007/s12010-020-03298-7 |
| [32] |
XU XD, HUANG CY, XU ZX, XU HX, WANG Z, YU XJ. The strategies to reduce cost and improve productivity in DHA production by Aurantiochytrium sp. : from biochemical to genetic respects[J]. Applied Microbiology and Biotechnology, 2020, 104(22): 9433-9447. DOI:10.1007/s00253-020-10927-y |
| [33] |
CHEN XH, SEN B, ZHANG S, BAI MH, HE YD, WANG GY. Chemical and physical culture conditions significantly influence the cell mass and docosahexaenoic acid content of Aurantiochytrium limacinum strain PKU#SW8[J]. Marine Drugs, 2021, 19(12): 671. DOI:10.3390/md19120671 |
| [34] |
QU L, REN LJ, SUN GN, JI XJ, NIE ZK, HUANG H. Batch, fed-batch and repeated fed-batch fermentation processes of the marine thraustochytrid Schizochytrium sp. for producing docosahexaenoic acid[J]. Bioprocess and Biosystems Engineering, 2013, 36(12): 1905-1912. DOI:10.1007/s00449-013-0966-7 |
| [35] |
WANG S, WAN WJ, WANG ZJ, ZHANG HD, LIU H, ARUNAKUMARA KKIU, CUI Q, SONG XJ. A two-stage adaptive laboratory evolution strategy to enhance docosahexaenoic acid synthesis in oleaginous thraustochytrid[J]. Frontiers in Nutrition, 2021, 8: 795491. DOI:10.3389/fnut.2021.795491 |
| [36] |
HAN X, LI ZH, WEN Y, CHEN Z. Overproduction of docosahexaenoic acid in Schizochytrium sp. through genetic engineering of oxidative stress defense pathways[J]. Biotechnology for Biofuels, 2021, 14(1): 70. DOI:10.1186/s13068-021-01918-w |
| [37] |
SHUIB S, NAZIR MYM, IBRAHIM I, SONG YD, RATLEDGE C, HAMID AA. Co-existence of type I fatty acid synthase and polyketide synthase metabolons in Aurantiochytrium SW1 and their implications for lipid biosynthesis[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2022, 1867(12): 159224. |
| [38] |
HEGGESET TMB, ERTESVÅG H, LIU B, ELLINGSEN TE, VADSTEIN O, AASEN IM. Lipid and DHA-production in Aurantiochytrium sp.- responses to nitrogen starvation and oxygen limitation revealed by analyses of production kinetics and global transcriptomes[J]. Scientific Reports, 2019, 9: 19470. DOI:10.1038/s41598-019-55902-4 |
| [39] |
MORABITO C, BOURNAUD C, MAËS C, SCHULER M, AIESE CIGLIANO R, DELLERO Y, MARÉCHAL E, AMATO A, RÉBEILLÉ F. The lipid metabolism in thraustochytrids[J]. Progress in Lipid Research, 2019, 76: 101007. DOI:10.1016/j.plipres.2019.101007 |
| [40] |
RATLEDGE C. Fatty acid biosynthesis in microorganisms being used for single cell oil production[J]. Biochimie, 2004, 86(11): 807-815. DOI:10.1016/j.biochi.2004.09.017 |
| [41] |
WANG FZ, BI YL, DIAO JJ, LÜ MM, CUI JY, CHEN L, ZHANG WW. Metabolic engineering to enhance biosynthesis of both docosahexaenoic acid and odd-chain fatty acids in Schizochytrium sp. S31[J]. Biotechnology for Biofuels, 2019, 12: 141. DOI:10.1186/s13068-019-1484-x |
| [42] |
LIANG YM, LIU Y, TANG J, MA J, CHENG JJ, DAROCH M. Transcriptomic profiling and gene disruption revealed that two genes related to PUFAs/DHA biosynthesis may be essential for cell growth of Aurantiochytrium sp.[J]. Marine Drugs, 2018, 16(9): 310. DOI:10.3390/md16090310 |
| [43] |
PRABHAKARAN P, RAETHONG N, NAZIR Y, HALIM H, YANG W, VONGSANGNAK W, HAMID AA, SONG YD. Whole genome analysis and elucidation of docosahexaenoic acid (DHA) biosynthetic pathway in Aurantiochytrium sp. SW1[J]. Gene, 2022, 846: 146850. DOI:10.1016/j.gene.2022.146850 |
| [44] |
LIPPMEIER JC, CRAWFORD KS, OWEN CB, RIVAS AA, METZ JG, APT KE. Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp.[J]. Lipids, 2009, 44(7): 621-630. DOI:10.1007/s11745-009-3311-9 |
| [45] |
HU F, CLEVENGER AL, ZHENG P, HUANG QY, WANG ZK. Low-temperature effects on docosahexaenoic acid biosynthesis in Schizochytrium sp. TIO01 and its proposed underlying mechanism[J]. Biotechnology for Biofuels, 2020, 13(1): 172. DOI:10.1186/s13068-020-01811-y |
| [46] |
DU F, WANG YZ, XU YS, SHI TQ, LIU WZ, SUN XM, HUANG H. Biotechnological production of lipid and terpenoid from thraustochytrids[J]. Biotechnology Advances, 2021, 48: 107725. DOI:10.1016/j.biotechadv.2021.107725 |
| [47] |
SHABALA L, McMEEKIN T, SHABALA S. Osmotic adjustment and requirement for sodium in marine protist thraustochytrid[J]. Environmental Microbiology, 2009, 11(7): 1835-1843. DOI:10.1111/j.1462-2920.2009.01908.x |
| [48] |
CHEN CY, LEE MH, DONG CD, LEONG YK, CHANG JS. Enhanced production of microalgal lipids using a heterotrophic marine microalga Thraustochytrium sp. BM2[J]. Biochemical Engineering Journal, 2020, 154: 107429. DOI:10.1016/j.bej.2019.107429 |
| [49] |
YOKOCHI T, HONDA D, HIGASHIHARA T, NAKAHARA T. Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21[J]. Applied Microbiology and Biotechnology, 1998, 49(1): 72-76. DOI:10.1007/s002530051139 |
| [50] |
CHEN W, ZHOU PP, ZHU YM, XIE C, MA L, WANG XP, BAO ZD, YU LJ. Improvement in the docosahexaenoic acid production of Schizochytrium sp. S056 by replacement of sea salt[J]. Bioprocess and Biosystems Engineering, 2016, 39(2): 315-321. DOI:10.1007/s00449-015-1517-1 |
| [51] |
UNAGUL P, ASSANTACHAI C, PHADUNGRUENGLUIJ S, PONGSUTEERAGUL T, SUPHANTHARIKA M, VERDUYN C. Biomass and docosahexaenoic acid formation by Schizochytrium mangrovei Sk-02 at low salt concentrations[J]. Botanica Marina, 2006, 49(2): 182-190. DOI:10.1515/BOT.2006.023 |
| [52] |
SUN XM, REN LJ, BI ZQ, JI XJ, ZHAO QY, HUANG H. Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis[J]. Bioresource Technology, 2018, 267: 438-444. DOI:10.1016/j.biortech.2018.07.079 |
| [53] |
UNAGUL P, ASSANTACHAI C, PHADUNGRUENGLUIJ S, SUPHANTHARIKA M, VERDUYN C. Properties of the docosahexaenoic acid-producer Schizochytrium mangrovei Sk-02: effects of glucose, temperature and salinity and their interaction[J]. Botanica Marina, 2005, 48(5/6): 387-394. |
| [54] |
HUANG JJH, CHEUNG PCK. Cold stress treatment enhances production of metabolites and biodiesel feedstock in Porphyridium cruentum via adjustment of cell membrane fluidity[J]. Science of the Total Environment, 2021, 780: 146612. DOI:10.1016/j.scitotenv.2021.146612 |
| [55] |
CUNHA E, SOUSA V, GEADA P, TEIXEIRA JA, VICENTE AA, DIAS O. Systems biology's role in leveraging microalgal biomass potential: current status and future perspectives[J]. Algal Research, 2023, 69: 102963. DOI:10.1016/j.algal.2022.102963 |
| [56] |
LIU J, GAO YY, JIANG XZ, MAO RY, TIAN BY, KE CR, WU SG, HUANG JZ. Effects on docosahexaenoic acid biosynthesis and expression of superoxide dismutase in Schizochytrium at low temperature[J]. Pharmaceutical Biotechnology, 2010, 17(1): 50-55. (in Chinese) 刘静, 高媛媛, 江贤章, 毛若雨, 田宝玉, 柯崇榕, 吴松刚, 黄建忠. 低温胁迫对裂殖壶菌DHA生物合成及SOD表达的影响[J]. 药物生物技术, 2010, 17(1): 50-55. DOI:10.19526/j.cnki.1005-8915.2010.01.008 |
| [57] |
ZENG Y, JI XJ, LIAN M, REN LJ, JIN LJ, OUYANG PK, HUANG H. Development of a temperature shift strategy for efficient docosahexaenoic acid production by a marine fungoid protist, Schizochytrium sp. HX-308[J]. Applied Biochemistry and Biotechnology, 2011, 164(3): 249-255. DOI:10.1007/s12010-010-9131-9 |
| [58] |
ZHANG ML, WANG J, WENG KX, LI L, HUANG JZ, LIN QQ. Docosahexaenoic acid production by Aurantiochytrium sp. FN21 under temperature shifting management[J]. Food and Fermentation Industries, 2022, 48(3): 15-19. (in Chinese) 张明亮, 王俊, 翁可欣, 李力, 黄建忠, 林清强. 变温调控破囊壶菌发酵生产二十二碳六烯酸[J]. 食品与发酵工业, 2022, 48(3): 15-19. DOI:10.13995/j.cnki.11-1802/ts.028351 |
| [59] |
YALI MP, JALILI H, NOROOZI M, MORADI Y, SABA F. Optimization of culture conditions for growth of the Aurantiochytrium sp. shy, isolated from the Persian Gulf[J]. Iranian Journal of Fisheries Sciences, 2019, 18(4): 656-671. |
| [60] |
ZHOU PP, LU MB, LI W, YU LJ. Microbial production of docosahexaenoic acid by a low temperature-adaptive strain Thraustochytriidae sp. Z105: screening and optimization[J]. Journal of Basic Microbiology, 2010, 50(4): 380-387. DOI:10.1002/jobm.200900378 |
| [61] |
ZHOU F, ZHANG ML, HUANG JZ, JIANG XZ. Effect of temperature shift on polyunsaturated fatty acids production and proteome of Thraustochytrium sp. FJN-10[J]. Biotechnology, 2015, 25(4): 384-390. (in Chinese) 周芬, 张明亮, 黄建忠, 江贤章. 变温对破囊壶菌FJN-10脂肪酸组分和蛋白质组的影响[J]. 生物技术, 2015, 25(4): 384-390. DOI:10.16519/j.cnki.1004-311x.2015.04.0078 |
| [62] |
REN LD, XU TH, XU QH, LIU SX, XIA W, ZHANG H. Screening and optimization of fermentation conditions of Schizochytrium strain with high-productive DHA[J]. China Oils and Fats, 2016, 41(5): 60-64. (in Chinese) 任良栋, 许团辉, 徐权汉, 刘思喜, 夏伟, 张浩. 一株高产DHA菌株的筛选及其发酵条件优化[J]. 中国油脂, 2016, 41(5): 60-64. |
| [63] |
LIU Y, TANG J, LI JJ, DAROCH M, CHENG JJ. Efficient production of triacylglycerols rich in docosahexaenoic acid (DHA) by osmo-heterotrophic marine protists[J]. Applied Microbiology and Biotechnology, 2014, 98(23): 9643-9652. DOI:10.1007/s00253-014-6032-9 |
| [64] |
ZHANG AQ, HE YD, SEN B, WANG WJ, WANG X, WANG GY. Optimal NaCl medium enhances squalene accumulation in Thraustochytrium sp. ATCC 26185 and influences the expression levels of key metabolic genes[J]. Frontiers in Microbiology, 2022, 13: 900252. DOI:10.3389/fmicb.2022.900252 |
| [65] |
LI L, TANG XY, LUO YY, HU XC, REN LJ. Accumulation and conversion of β-carotene and astaxanthin induced by abiotic stresses in Schizochytrium sp.[J]. Bioprocess and Biosystems Engineering, 2022, 45(5): 911-920. DOI:10.1007/s00449-022-02709-9 |
| [66] |
ZHU LY, ZHANG XC, JI L, SONG XJ, KUANG CH. Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities[J]. Process Biochemistry, 2007, 42(2): 210-214. DOI:10.1016/j.procbio.2006.08.002 |
| [67] |
TAOKA Y, NAGANO N, OKITA Y, IZUMIDA H, SUGIMOTO S, HAYASHI M. Influences of culture temperature on the growth, lipid content and fatty acid composition of Aurantiochytrium sp. strain mh0186[J]. Marine Biotechnology, 2009, 11(3): 368-374. DOI:10.1007/s10126-008-9151-4 |
| [68] |
PAREDES P, LARAMA G, FLORES L, LEYTON A, ILI CG, ASENJO JA, CHISTI Y, SHENE C. Temperature differentially affects gene expression in Antarctic thraustochytrid Oblongichytrium sp. RT2316-13[J]. Marine Drugs, 2020, 18(11): 563. DOI:10.3390/md18110563 |
| [69] |
SHENE C, PAREDES P, FLORES L, LEYTON A, ASENJO JA, CHISTI Y. Dynamic flux balance analysis of biomass and lipid production by Antarctic thraustochytrid Oblongichytrium sp. RT2316-13[J]. Biotechnology and Bioengineering, 2020, 117(10): 3006-3017. DOI:10.1002/bit.27463 |
| [70] |
MA ZX, TAN YZ, CUI GZ, FENG YG, CUI Q, SONG XJ. Transcriptome and gene expression analysis of DHA producer Aurantiochytrium under low temperature conditions[J]. Scientific Reports, 2015, 5: 14446. DOI:10.1038/srep14446 |
| [71] |
YE HK. Thriving for higher yield of DHA through fermentation technology in Thraustochytriidae sp. PKU#Mn16[D]. Tianjin: Doctoral Dissertation of Tianjin University, 2020 (in Chinese). 叶会科. 破囊壶菌Thraustochytriidae sp. PKU#Mn16高产DHA的发酵技术研究[D]. 天津: 天津大学博士学位论文, 2020. |
| [72] |
BI ZQ, REN LJ, HU XC, SUN XM, ZHU SY, JI XJ, HUANG H. Transcriptome and gene expression analysis of docosahexaenoic acid producer Schizochytrium sp. under different oxygen supply conditions[J]. Biotechnology for Biofuels, 2018, 11: 249. DOI:10.1186/s13068-018-1250-5 |
| [73] |
CHANG GF, WU J, JIANG CH, TIAN GW, WU QH, CHANG M, WANG XG. The relationship of oxygen uptake rate and kLa with rheological properties in high cell density cultivation of docosahexaenoic acid by Schizochytrium sp. S31[J]. Bioresource Technology, 2014, 152: 234-240. DOI:10.1016/j.biortech.2013.11.002 |
| [74] |
GUO DS, JI XJ, REN LJ, LI GL, YIN FW, HUANG H. Development of a real-time bioprocess monitoring method for docosahexaenoic acid production by Schizochytrium sp.[J]. Bioresource Technology, 2016, 216: 422-427. DOI:10.1016/j.biortech.2016.05.044 |
| [75] |
QU L, JI XJ, REN LJ, NIE ZK, FENG Y, WU WJ, OUYANG PK, HUANG H. Enhancement of docosahexaenoic acid production by Schizochytrium sp. using a two-stage oxygen supply control strategy based on oxygen transfer coefficient[J]. Letters in Applied Microbiology, 2011, 52(1): 22-27. DOI:10.1111/j.1472-765X.2010.02960.x |
| [76] |
CIPAK A, HASSLACHER M, TEHLIVETS O, COLLINSON EJ, ZIVKOVIC M, MATIJEVIC T, WONISCH W, WAEG G, DAWES IW, ZARKOVIC N, KOHLWEIN SD. Saccharomyces cerevisiae strain expressing a plant fatty acid desaturase produces polyunsaturated fatty acids and is susceptible to oxidative stress induced by lipid peroxidation[J]. Free Radical Biology and Medicine, 2006, 40(5): 897-906. DOI:10.1016/j.freeradbiomed.2005.10.039 |
| [77] |
CHI ZY, LIU Y, FREAR C, CHEN SL. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level[J]. Applied Microbiology and Biotechnology, 2009, 81(6): 1141-1148. DOI:10.1007/s00253-008-1740-7 |
| [78] |
LIU L, ZHU XY, YE HK, WEN YY, SEN B, WANG GY. Low dissolved oxygen supply functions as a global regulator of the growth and metabolism of Aurantiochytrium sp. PKU#Mn16 in the early stages of docosahexaenoic acid fermentation[J]. Microbial Cell Factories, 2023, 22(1): 52. DOI:10.1186/s12934-023-02054-w |
| [79] |
JAKOBSEN AN, AASEN IM, JOSEFSEN KD, STRØM AR. Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: effects of N and P starvation and O2 limitation[J]. Applied Microbiology and Biotechnology, 2008, 80(2): 297-306. DOI:10.1007/s00253-008-1537-8 |
| [80] |
AASEN IM, ERTESVÅG H, HEGGESET TMB, LIU B, BRAUTASET T, VADSTEIN O, ELLINGSEN TE. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids[J]. Applied Microbiology and Biotechnology, 2016, 100(10): 4309-4321. DOI:10.1007/s00253-016-7498-4 |
| [81] |
MIEDEMA H, STAAL M, PRINS HA. pH-induced proton permeability changes of plasma membrane vesicles[J]. The Journal of Membrane Biology, 1996, 152(2): 159-167. DOI:10.1007/s002329900094 |
| [82] |
AABO T, GLÜCKSTAD J, SIEGUMFELDT H, ARNEBORG N. Intracellular pH distribution as a cell health indicator in Saccharomyces cerevisiae[J]. Journal of the Royal Society, Interface, 2011, 8(64): 1635-1643. DOI:10.1098/rsif.2011.0148 |
| [83] |
WU ST, YU ST, LIN LP. Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31[J]. Process Biochemistry, 2005, 40(9): 3103-3108. DOI:10.1016/j.procbio.2005.03.007 |
| [84] |
ZHAO XY, REN LJ, GUO DS, WU WJ, JI XJ, HUANG H. CFD investigation of Schizochytrium sp. impeller configurations on cell growth and docosahexaenoic acid synthesis[J]. Bioprocess and Biosystems Engineering, 2016, 39(8): 1297-1304. DOI:10.1007/s00449-016-1608-7 |
| [85] |
ZHAO B, LI YF, MBIFILE MD, LI CL, YANG HL, WANG W. Improvement of docosahexaenoic acid fermentation from Schizochytrium sp. AB-610 by staged pH control based on cell morphological changes[J]. Engineering in Life Sciences, 2017, 17(9): 981-988. DOI:10.1002/elsc.201600249 |
| [86] |
SHAFIQ M, ZEB L, CUI GN, JAWAD M, CHI ZY. High-density pH-auxostat fed-batch culture of Schizochytrium limacinum SR21 with acetic acid as a carbon source[J]. Applied Biochemistry and Biotechnology, 2020, 192(4): 1163-1175. DOI:10.1007/s12010-020-03396-6 |
| [87] |
YIN FW, ZHANG YT, JIANG JY, GUO DS, GAO S, GAO Z. Efficient docosahexaenoic acid production by Schizochytrium sp. via a two-phase pH control strategy using ammonia and citric acid as pH regulators[J]. Process Biochemistry, 2019, 77: 1-7. DOI:10.1016/j.procbio.2018.11.013 |
| [88] |
BOURAS S, ANTONIADIS D, KOUNTRIAS G, KARAPANAGIOTIDIS IT, KATSOULAS N. Effect of pH on Schizochytrium limacinum production grown using crude glycerol and biogas digestate effluent[J]. Agronomy, 2022, 12(2): 364. DOI:10.3390/agronomy12020364 |
| [89] |
KHUMRANGSEE K, CHAROENRAT T, PRAIBOON J, CHITTAPUN S. Development of a fed-batch fermentation with stepwise aeration to enhance docosahexaenoic acid and carotenoid content in Aurantiochytrium sp. FIKU018[J]. Journal of Applied Phycology, 2022, 34(3): 1243-1253. DOI:10.1007/s10811-022-02726-x |
| [90] |
KAYA K, KAZAMA Y, ABE T, SHIRAISHI F. Influence of medium components and pH on the production of odd-carbon fatty acids by Aurantiochytrium sp. SA-96[J]. Journal of Applied Phycology, 2020, 32(3): 1597-1606. DOI:10.1007/s10811-020-02111-6 |
| [91] |
WANG SK, WANG X, TIAN YT, CUI YH. Nutrient recovery from tofu whey wastewater for the economical production of docosahexaenoic acid by Schizochytrium sp. S31[J]. Science of the Total Environment, 2020, 710: 136448. DOI:10.1016/j.scitotenv.2019.136448 |
| [92] |
YIN Y, DING Y, FENG G, LI J, XIAO L, KARUPPIAH V, SUN W, ZHANG F, LI Z. Modification of artificial sea water for the mass production of (+)-terrein by Aspergillus terreus strain PF26 derived from marine sponge Phakellia fusca[J]. Letters in Applied Microbiology, 2015, 61(6): 580-587. DOI:10.1111/lam.12496 |
| [93] |
ZHANG AQ, MERNITZ K, WU C, XIONG W, HE YD, WANG GY, WANG X. ATP drives efficient terpene biosynthesis in marine thraustochytrids[J]. mBio, 2021, 12(3): e00881-21. |
| [94] |
ZHANG AQ, XIE YX, HE YD, WANG WJ, SEN B, WANG GY. Bio-based squalene production by Aurantiochytrium sp. through optimization of culture conditions, and elucidation of the putative biosynthetic pathway genes[J]. Bioresource Technology, 2019, 287: 121415. DOI:10.1016/j.biortech.2019.121415 |
| [95] |
SHABALA L, McMEEKIN T, SHABALA S. Thraustochytrids can be grown in low-salt media without affecting PUFA production[J]. Marine Biotechnology, 2013, 15(4): 437-444. DOI:10.1007/s10126-013-9499-y |
| [96] |
WANG S, LYU XY, ZHANG JW, TIAN H, CHEN T, HE DP. Impacts of different concentrations of carbon and nitrogen sources and culture temperature on DHA production by Schizochytrium sp.[J]. China Oils and Fats, 2015, 40(10): 74-77. (in Chinese) 王澍, 吕小义, 张静雯, 田华, 陈涛, 何东平. 不同碳氮源浓度和培养温度对裂殖壶菌产DHA的影响[J]. 中国油脂, 2015, 40(10): 74-77. |
| [97] |
WANG JP, LUO Y, XI DX. Effects of agitation and aeration on DHA production by Schizochytrium LX0809[J]. Chinese Journal of Bioprocess Engineering, 2017, 15(1): 16-19. (in Chinese) 王杰鹏, 罗瑶, 郗大兴. 搅拌和通气对裂殖壶菌LX0809产DHA的影响[J]. 生物加工过程, 2017, 15(1): 16-19. |
| [98] |
REN LJ, JI XJ, HUANG H, QU L, FENG Y, TONG QQ, OUYANG PK. Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp.[J]. Applied Microbiology and Biotechnology, 2010, 87(5): 1649-1656. DOI:10.1007/s00253-010-2639-7 |
| [99] |
ZHANG ML, WU WB, GUO XL, YOU WC, QI F, JIANG XZ, HUANG JZ. Mathematical modeling of fed-batch fermentation of Schizochytrium sp. FJU-512 growth and DHA production using a shift control strategy[J]. 3 Biotech, 2018, 8: 162. |
| [100] |
CHI GX, XU YY, CAO XY, LI ZP, CAO MF, CHISTI Y, HE N. Production of polyunsaturated fatty acids by Schizochytrium (Aurantiochytrium) spp.[J]. Biotechnology Advances, 2022, 55: 107897. DOI:10.1016/j.biotechadv.2021.107897 |
| [101] |
SUN XM, REN LJ, BI ZQ, JI XJ, ZHAO QY, JIANG L, HUANG H. Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp.[J]. Biotechnology for Biofuels, 2018, 11: 65. DOI:10.1186/s13068-018-1065-4 |
| [102] |
SUN XM, GENG LJ, REN LJ, JI XJ, HAO N, CHEN KQ, HUANG H. Influence of oxygen on the biosynthesis of polyunsaturated fatty acids in microalgae[J]. Bioresource Technology, 2018, 250: 868-876. DOI:10.1016/j.biortech.2017.11.005 |
| [103] |
ARORA N, YEN HW, PHILIPPIDIS GP. Harnessing the power of mutagenesis and adaptive laboratory evolution for high lipid production by oleaginous microalgae and yeasts[J]. Sustainability, 2020, 12(12): 5125. DOI:10.3390/su12125125 |
| [104] |
MAVROMMATI M, DASKALAKI A, PAPANIKOLAOU S, AGGELIS G. Adaptive laboratory evolution principles and applications in industrial biotechnology[J]. Biotechnology Advances, 2022, 54: 107795. DOI:10.1016/j.biotechadv.2021.107795 |
| [105] |
HU XC, TANG XY, BI ZQ, ZHAO QY, REN LJ. Adaptive evolution of microalgae Schizochytrium sp. under high temperature for efficient production of docosahexaeonic acid[J]. Algal Research, 2021, 54: 102212. DOI:10.1016/j.algal.2021.102212 |
| [106] |
SUN XM, REN LJ, JI XJ, CHEN SL, GUO DS, HUANG H. Adaptive evolution of Schizochytrium sp. by continuous high oxygen stimulations to enhance docosahexaenoic acid synthesis[J]. Bioresource Technology, 2016, 211: 374-381. DOI:10.1016/j.biortech.2016.03.093 |
| [107] |
REN LJ, SUN XM, JI XJ, CHEN SL, GUO DS, HUANG H. Enhancement of docosahexaenoic acid synthesis by manipulation of antioxidant capacity and prevention of oxidative damage in Schizochytrium sp.[J]. Bioresource Technology, 2017, 223: 141-148. DOI:10.1016/j.biortech.2016.10.040 |
| [108] |
BAO ZD, ZHU YM, FENG YM, ZHANG K, ZHANG M, WANG ZK, YU LJ. Enhancement of lipid accumulation and docosahexaenoic acid synthesis in Schizochytrium sp. H016 by exogenous supplementation of sesamol[J]. Bioresource Technology, 2022, 345: 126527. DOI:10.1016/j.biortech.2021.126527 |
| [109] |
SINGH D, MATHUR AS, TULI DK, PURI M, BARROW CJ. Propyl gallate and butylated hydroxytoluene influence the accumulation of saturated fatty acids, omega-3 fatty acid and carotenoids in thraustochytrids[J]. Journal of Functional Foods, 2015, 15: 186-192. DOI:10.1016/j.jff.2015.03.022 |
| [110] |
GAFFNEY M, O՚ROURKE R, MURPHY R. Manipulation of fatty acid and antioxidant profiles of the microalgae Schizochytrium sp. through flaxseed oil supplementation[J]. Algal Research, 2014, 6: 195-200. DOI:10.1016/j.algal.2014.03.005 |
| [111] |
YU XJ, SUN J, SUN YQ, ZHENG JY, WANG Z. Metabolomics analysis of phytohormone gibberellin improving lipid and DHA accumulation in Aurantiochytrium sp.[J]. Biochemical Engineering Journal, 2016, 112: 258-268. DOI:10.1016/j.bej.2016.05.002 |
| [112] |
ZHANG S, HE YD, SEN B, CHEN XH, XIE YX, KEASLING JD, WANG GY. Alleviation of reactive oxygen species enhances PUFA accumulation in Schizochytrium sp. through regulating genes involved in lipid metabolism[J]. Metabolic Engineering Communications, 2018, 6: 39-48. DOI:10.1016/j.meteno.2018.03.002 |
| [113] |
POLBRAT T, KONKOL D, KORCZYNSKI M. Optimization of docosahexaenoic acid production by Schizochytrium sp. : a review[J]. Biocatalysis and Agricultural Biotechnology, 2021, 35: 102076. DOI:10.1016/j.bcab.2021.102076 |
 2023, Vol. 50
2023, Vol. 50