扩展功能
文章信息
- 田颖, 张娜娜, 秦成峰, 李晓峰
- TIAN Ying, ZHANG Nana, QIN Chengfeng, LI Xiaofeng
- mRNA技术应对病毒传染病的研究进展
- Advancements in mRNA technology-based therapies for infectious diseases
- 微生物学通报, 2022, 49(7): 2849-2861
- Microbiology China, 2022, 49(7): 2849-2861
- DOI: 10.13344/j.microbiol.china.210982
-
文章历史
- 收稿日期: 2021-10-20
- 接受日期: 2021-12-09
- 网络首发日期: 2022-01-21
2. 军事科学院军事医学研究院微生物流行病研究所 病原微生物生物安全国家重点实验室, 北京 100071;
3. 清华大学医学院, 北京 100084
2. State Key Laboratory of Pathogen and Biosecurity, Institute of Microbiology and Epidemiology, Academy of Military Medicine, Academy of Military Sciences, Beijing 100071, China;
3. School of Medicine, Tsinghua University, Beijing 100084, China
不断暴发的新突发病毒传染病疫情严重威胁人类生命健康,也给全球经济带来巨大冲击和重大损失。疫苗是应对传染病最有效的手段,但传统疫苗研发周期长、成本高,在应对新突发传染病时更显力不从心。mRNA疫苗的概念形成于20世纪90年代[1],其安全性好、有效性高、设计灵活、研发周期短,一直是疫苗领域中的重点攻关方向。但因为一直未能解决核酸降解和高效递送等难题,所以mRNA疫苗的进展缓慢。近10年来,基因修饰和体外递送等技术不断迭代更新,mRNA技术领域持续取得重要突破,在传染病预防、肿瘤治疗方面显现出广阔的应用前景。特别是新型冠状病毒mRNA疫苗以破纪录的速度完成研发,更是为mRNA疫苗在未来的推广使用铺平了道路。本文主要综述最近5年mRNA技术及其在应对传染病方面的重要进展,并尝试对该技术未来在应对新突发病毒传染病领域的发展趋势进行展望。
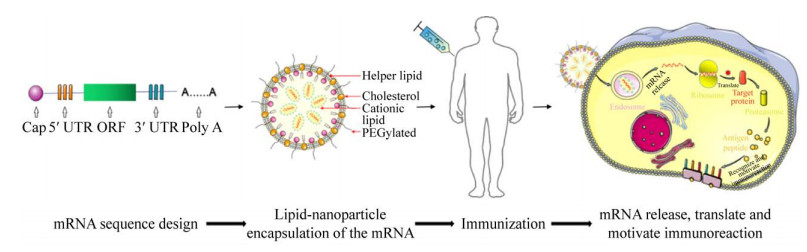
1 mRNA技术 1.1 mRNA技术的原理与优势mRNA技术概念的出现并形成是在20世纪90年代。1990年Wolff等将经由体外转录获得的mRNA直接注入小鼠肌肉后发现,其编码的氯霉素乙酰转移酶能够在肌肉细胞中有效表达[1]。1992年Jirikowski等进一步揭示,将编码抗利尿激素的mRNA注射至大鼠下丘脑后能使大鼠产生特异的生理反应[2]。科学家据此提出基于mRNA技术的新型疫苗策略(图 1),其原理是将编码抗原蛋白的mRNA导入细胞质,利用细胞内蛋白合成组件生成抗原,进而激活机体的免疫系统来应对病原体感染[3-4]。mRNA疫苗的类型主要包括非复制型和自我扩增型。前者仅包括靶抗原序列,通过体外转录体系获得mRNA分子,后者则是将病毒的结构基因替换为目的蛋白基因,进一步将重组病毒载体转入细胞,利用病毒高效的基因组复制能力合成大量目的蛋白的mRNA[5]。
|
| 图 1 mRNA疫苗技术原理 Figure 1 Principle of mRNA vaccine technology. |
|
|
与传统疫苗和DNA疫苗相比,mRNA疫苗在安全性、有效性和产能方面具有明显优势(表 1)[6-8],首先是安全性高,其整合至受体基因组的风险极低;其次是有效性好,能同时激活细胞免疫和体液免疫等均衡的免疫应答;更为重要的是,mRNA抗原设计灵活、制备工艺简单、产能可迅速放大,在应对新突发病毒传染病时优势尽显。
| Vaccine type | Safety | Validity | Manufacturing |
| mRNA vaccine | No infection; Very low insertion mutation ris; In vivo activity can be controlled, through the normal cellular pathway can be degraded | Similar or better immune protection than existing vaccines | Encoding any new antigen requires no cell culture and can be rapidly scaled up at low cost |
| DNA vaccine | There is a risk of integration into the recipient genome and no risk of recovery of pathogenicity | Low immunogenicity | Short production cycle, low cost, can be large-scale production |
| Inactivated vaccine | Safer | The immune protection effect is generally lower than live attenuated vaccine | Dependent on cell culture production, long cycle and high cost |
| Live attenuated vaccine | There are risks of virulence recovery, recombination with wild strains and production contamination | It has strong immune protection effect | Dependent on cell culture production, long cycle and high cost |
| Subunit vaccine | Safer; Less side effects | Poor immunogenicity, the use of adjuvant to enhance the immune effect | Rapid preparation, complex production procedure and high cost |
真核生物的mRNA分子依次由5′端帽子、5′非编码区、编码区、3′非编码区和多聚腺苷酸尾组成,5′端帽子是N7甲基化的鸟苷酸,通过5′-ppp与mRNA的5′端核苷酸连接形成m7GpppN,通过结合翻译起始因子来保护mRNA不被核酸外切酶降解,同时协助mRNA起始翻译和避免被天然免疫系统识别[9-10];5′非编码区含有核糖体识别序列,与3′非编码区协同起始mRNA的翻译进程,并完成mRNA的亚细胞定位;3′末端的多聚腺苷酸主要维持mRNA的稳定[11]。
体外转录生成的mRNA分子稳定性差、翻译效率低,一直是mRNA疫苗研发过程中需要重点解决的技术难题,研究者相继开发了一系列mRNA分子合成和修饰等技术,上述问题也得到极大改观。获得天然的5′端帽子是mRNA行使功能的根本前提,传统加帽过程多采用共转录技术完成,即通过将抗逆转帽类似物(anti-reverse cap analogues,ARCA)与GTP按照一定比例混合后,通过体外转录反应在合成mRNA过程中完成加帽[12],但该方法的加帽效率仅能达到80%,并且产量仅为1 mg/mL[13]。2017年,TriLink公司开发了下一代加帽技术CleanCap系统,加帽效率可提高至94%以上,产量提升至5–10 mg/mL,已成为绝大部分mRNA技术公司的首选[13-14]。5′和3′非编码区主要影响编码蛋白的表达和mRNA的半衰期[15-16],匹配合理的非编码区组合也是提高mRNA翻译效率的关键步骤。Sample等通过构建30 000条能够表达报告基因的随机5′非编码区序列,建立了能够预测5′非编码区起始翻译效率的模型,大大便利了高效5′非编码序列的筛选[17]。针对3′非编码区的设计优化也取得多项进展,如通过增加该区的拷贝数可有效提升mRNA的表达效率,还可通过消除该区目标细胞特异的miRNA靶序列来避免mRNA被靶细胞快速降解[16],或通过引入非目标细胞特异miRNA的靶序列,定向降低mRNA在非靶细胞中的蛋白表达[18-19],有力提升了mRNA分子表达的特异性。此外,缩短非编码区中阻碍核糖体进入的特定序列长度也能提高mRNA的翻译效率[20]。目前mRNA技术公司多选用β珠蛋白(β-globin)的非编码区来提高mRNA的稳定性和表达效率[21]。mRNA的编码序列一般需根据实际情况进行密码子优化,以提高编码蛋白的翻译效率,也可通过增加其GC含量来提高mRNA的稳定性[22-23]。近期研究人员还开发了一种通过最大化碱基堆叠策略优化mRNA序列的算法,经过该算法获得的mRNA序列的稳定性显著提高[24]。多数mRNA末端的多聚腺苷酸数目大于90,但腺苷酸的数量对mRNA稳定性的影响尚无定论,如Lima等的研究显示,翻译效率高的mRNA实际上仅含33–34个腺苷酸尾[25],但另一项研究发现人的原代T细胞里高效表达的mRNA的多聚腺苷酸数量超过300[26]。多聚腺苷酸及其数量如何调控体外转录体mRNA的表达效率、是否具有细胞特异性是未来重点关注的研究方向。
体外合成的mRNA分子相对细胞具有异质性,极易刺激细胞产生Ⅰ型干扰素为主的天然免疫应答,进而会被宿主细胞迅速清除,因此,其也是制约mRNA有效发挥功能的关键因素之一。研究显示,通过引入假尿嘧啶、N1-甲基化假尿嘧啶及其他类型的核酸类似物能有效降低宿主的病原模式识别受体对mRNA分子的甄别能力,进而显著提高蛋白的表达效率[11]。Moderna公司研发的新型冠状病毒mRNA疫苗mRNA-1273、Pfizer和BioNTech公司共同研发的新型冠状病毒mRNA疫苗BNT162b1及BNT162b2序列中均引入了假尿嘧啶、N1-甲基假尿嘧啶等修饰,mRNA的翻译效率显著提高[27-28]。CureVac公司则通过降低尿嘧啶比例、提高GC含量的策略确保mRNA序列能够逃避免疫系统的识别[22]。此外,以m6A等为代表的转录后表观遗传修饰参与调控免疫系统对RNA分子的识别和应答过程,研究显示RNA甲基转移酶mettl3能有效激活树突状细胞,促进基于树突状细胞的T细胞免疫应答[29],提示将该酶的mRNA与目标mRNA共转染有望改善mRNA分子引发的免疫应答。
1.3 mRNA的递送方式如何将mRNA高效递送至细胞内并避免被降解是mRNA疫苗研发面临的另一项关键技术瓶颈。研究人员尝试了包括鱼精蛋白、多糖蛋白、阳离子聚合物、脂质纳米颗粒(lipid nanoparticle,LNP)等多种mRNA递送系统[30-31],但早期的多数材料因本身毒性高、结构复杂、聚合度不可控等缺点未能进入临床研究。LNP具有细胞相容性好、免疫原性低、核酸包封率高等优势,是目前研究最深入且应用最广泛的mRNA递送系统。LNP由可电离脂质、磷脂、聚乙二醇(polyethylene glycol,PEG)修饰脂质和胆固醇四部分组成[32],可电离脂质通过不同的pH环境调节载体自组装和mRNA的释放,磷脂是脂质双层结构的核心组分,PEG修饰脂质用于延长mRNA的半衰期,胆固醇则有助于提高颗粒稳定性并协助颗粒与细胞膜发生融合[33]。研究人员还尝试多种策略赋予了LNP新的功能,例如多环金刚烷尾或环状咪唑头可显著提高LNP靶向T细胞的特异性[34],而连接有环胺基团的LNP可与干扰素刺激蛋白紧密结合,进而促进树突状细胞成熟,发挥潜在的抗肿瘤作用[35]。
聚合物纳米颗粒是除LNP外的另一大类核酸递送载体。聚乙烯亚胺递送核酸的效率极高,进一步通过引入PEG、连接环糊精等方式显著降低了其毒性[36-37],也促使聚乙烯亚胺成为聚合物核酸递送载体的典型代表。此外,研究人员还开发了多聚β-氨基酯等可生物降解的低毒性聚合物[38-39],并且获得了可对pH环境做出响应的乙烯亚胺-多聚天冬酰胺聚合物载体[40],其在动物体内显示出高效的递送和释放效率[41-42]。
1.4 mRNA抗体技术单抗免疫疗法在自身免疫疾病、神经退行性疾病、抗肿瘤、传染病治疗方面发挥了重要作用,而重组抗体技术、抗体分选与抗体基因测序等领域取得的一系列突破,则有力促进并催生了一批单抗获批上市[43-45]。然而传统的单抗研发制备周期长、生产成本昂贵,迫切需要开发下一代抗体制备新技术,mRNA抗体技术随之应运而生。与mRNA疫苗的设计原理一致,mRNA抗体技术是将编码抗体轻链和重链的mRNA递送至细胞内,利用细胞机器合成组装成结构相同并具有功能活性的目标抗体,进而在体内发挥相应的治疗功能。研究发现,mRNA抗体制剂注入体内2 h后即可检测到抗体[46],而DNA或者腺病毒载体递送的抗体制剂在注射后3–7 d后才能表达出抗体[47-48]。mRNA抗体在体内的表达峰值大约出现在注射后24–48 h,并可持续高水平表达长达数天[46, 49]。mRNA抗体在体内表达峰值所需时间更短且持续时间更长,在发挥抗体疗效方面将更具优势。此外,mRNA技术特有的设计灵活和产能易扩的特点也赋予mRNA抗体独特的应用优势,特别是在应对新突发传染病方面将能够迅速完成从抗体设计到制剂制备等一系列工作,为临床救治赢得宝贵时间[50]。
2 mRNA技术在应对病毒传染病中的应用 2.1 新型冠状病毒mRNA疫苗冠状病毒的刺突蛋白(spike,S)是病毒最主要的免疫原,其中的受体结合域(receptor binding domain,RBD)是与中和抗体结合的关键区域,因此也是新型冠状病毒疫苗设计的首选靶标[33]。新型冠状病毒mRNA疫苗研发的头部企业是BioNTech和Moderna公司。BioNTech研发的两款新型冠状病毒mRNA疫苗BNT162b1和BNT162b2进展最快,前者编码RBD,并辅以T4纤维蛋白结构域而形成具有更强免疫原性的三聚体[51],后者编码全长S蛋白,同时通过引入2个脯氨酸突变锁定S蛋白的膜融合前构象,便于诱导产生特异性更高的中和抗体[28],二者均采用LNP完成mRNA分子的体内递送。一期临床试验数据显示,BNT162b1和BNT162b2均具有良好的安全性,除BNT162b1的不良反应率稍高外,二者均可诱导人体产生相近的极高水平的中和抗体和强烈的CD4+和CD8+细胞反应[52-53],三期临床试验的结果显示BNT162b2对新型冠状病毒感染的保护效力达到惊人的94.6%[28]。该疫苗也于2020年12月中旬率先获得美国食品药品监督管理局(Food and Drug Administration,FDA)的紧急使用授权。之后在以色列开展的涵盖300多万人群的疫苗保护效力监测数据显示,BNT162b2在真实世界的有效率维持在90%以上[54]。基于其良好的有效性和安全性,BNT162b2于2021年8月23日正式获美国FDA批准用于16岁及以上人群预防新型冠状病毒肺炎,成为首个获批正式上市的新型冠状病毒疫苗,但该疫苗要求在不高于–60 ℃条件下储存和运输,限制了其在经济落后地区的推广使用。Moderna公司研发的新型冠状病毒mRNA疫苗mRNA-1273编码全长S蛋白,同样采用了双脯氨酸突变方式维持S蛋白的膜融合前构象,并通过LPN完成mRNA的递送。三期临床试验的结果显示其有效性达94.1%,而且完成接种6个月后仍能检测到抗体[27]。此外,mRNA-1273无需超低温储存,可以在–20 ℃保存6个月,在2–8 ℃保存30 d[55-56],也继BNT162b2后于2020年12月中旬获美国FDA的紧急使用授权。特别指出的是,该疫苗从序列设计开始到启动一期临床试验仅用了66 d,充分展现了mRNA技术在应对突发传染病中所具备的研发迅速这一突出优势[33]。我国在2020年1月底启动了新型冠状病毒mRNA疫苗研发项目,进展最快的是由军事科学院联合多家企业共同研发的ARCoV。该疫苗的mRNA编码RBD,使用以具有自主知识产权的阳离子脂质9001为组分的LNP完成mRNA的体内递送,ARCoV在小鼠和非灵长类动物体内可刺激产生高水平的中和抗体和Th1偏向的T细胞免疫反应,发挥均衡的体液和细胞免疫保护作用。更为突出的是,ARCoV可在室温条件下保持活性至少一周,热稳定性优异[57]。一期临床试验的结果表明,ARCoV免疫人群后仅出现一过性发热等症状,安全耐受性好,更为重要的是免疫人群产生高水平的新型冠状病毒中和抗体和强烈的细胞免疫反应[58]。ARCoV已于2021年7月在中国云南和广西启动临床三期试验,9月在尼泊尔和墨西哥进入海外临床三期研究阶段。
鉴于新型冠状病毒mRNA疫苗率先启动大规模人群接种,并展现出优异的保护效力,研究人员得以对真实世界中人群的免疫应答规律和持久性、疫苗的安全性、序贯免疫的应答特征[59-60]、疫苗对新型冠状病毒须关切变种(variant of concern,VOC)的中和能力和保护效果[61-63]等开展全面、深入的研究。在免疫应答机制方面,Amanat等比较了新型冠状病毒康复者与疫苗接种健康者的体液免疫反应情况,发现BNT162b2免疫人体后能产生与康复者相当或更高的针对RBD和N端域(N-terminal domain,NTD)的抗体,但疫苗免疫组无中和活性的抗体比例更高[64]。Painter等监测了疫苗接种健康者体内T细胞的动态变化情况,发现首针免疫即可迅速激发S蛋白特异的CD4+ T细胞应答,其中Th1和Tfh型T细胞分别有助于二针免疫后迅速产生CD8+ T细胞和中和抗体[65]。此外,Ciabattini等[66]和Woldemeskel等[67]发现,BNT162b2免疫产生的S蛋白特异的记忆性B细胞能维持6个月以上高水平[66],而T细胞整体水平虽在6个月后有所下降,但对S蛋白多肽的刺激仍能保持较高活性[67],提示完成接种6个月后人体仍能对病毒感染迅速做出响应,从而建立有效的免疫保护状态,这为疫苗加强接种的必要性提供了重要依据。新型冠状病毒mRNA疫苗在全球接种量已超数十亿剂,其良好的安全性也得到了充分认可。JAMA发表的一项涵盖620万名疫苗接种者的监测数据显示,接种新型冠状病毒mRNA疫苗后1–21 d内未出现23种潜在的严重不良事件[68]。目前的研究也显示,接种新型冠状病毒mRNA疫苗未增加孕妇出现不良妊娠的风险[69]。但需指出的是,新型冠状病毒mRNA疫苗接种可能引发心肌炎,发生率约为2.13例/10万人,特别是在16–29岁的男性接种者中比例更高,约为10.49例/10万人[70-71]。此外,mRNA疫苗还可能引发极个别的免疫血栓性血小板减少症病例,也需持续关注[72]。上述研究极大地加深了我们对mRNA疫苗免疫保护机制的认识,为未来制定有效的疫苗接种策略、指导下一代mRNA疫苗的研发提供了全面、丰富的科学依据。
2.2 寨卡病毒mRNA疫苗寨卡病毒(Zika virus,ZIKV)是一种经蚊虫叮咬传播的黄病毒,但2015年起暴发于南美洲的寨卡病毒被证实还能经血液、体液传播,并可导致新生儿小头症,彻底颠覆了人们对虫媒病毒的固有认知[73],研发有效的寨卡病毒疫苗成为当前寨卡相关研究的焦点。虫媒黄病毒的膜蛋白前体prM和包膜蛋白E是中和抗体的主要结合区,因此也是疫苗研发的关键靶标。2017年2月宾夕法尼亚大学的Pardi等发表了首个寨卡病毒mRNA疫苗的临床前研究结果,该疫苗选取2013年寨卡病毒流行株的prM和E蛋白基因作为mRNA的编码序列,并引入1-甲基假尿苷(M1Ψ),将经修饰的mRNA用LNP递送至小鼠和恒河猴的皮内后,均能诱导产生强大和持久的中和抗体[74]。与此同时,Moderna公司联合华盛顿大学医学院,通过在E蛋白融合环表位中引入突变,设计开发了寨卡病毒mRNA疫苗mRNA-1893,其不仅能诱导产生针对寨卡病毒的特异性保护抗体,同时能够减少增强登革病毒感染的交叉反应抗体的产生[75]。该疫苗也率先进入一期临床试验,其中的数据显示,10 μg和30 μg的mRNA-1893可诱导94%−100%的血清阳转率,并具有良好的耐受性[76]。mRNA-1893的相关研究发现再次展现了mRNA疫苗设计简便、快速、有效的优势,同时为设计能降低抗体依赖的感染增强作用的登革病毒mRNA疫苗提供了重要借鉴。寨卡病毒mRNA疫苗诱发产生的中和抗体水平显著高于传统疫苗,并能有效激活保护性的T细胞免疫应答,有望在未来发展为应对寨卡病毒感染的高效手段。
2.3 流感病毒mRNA疫苗现有流感疫苗的主要保护性抗原组分是病毒的血凝素蛋白,但该蛋白极易发生抗原漂移,每年必须针对可能的新流行株调整和更新流感疫苗,此外病毒在鸡胚生产过程中积累的适应性突变也可能影响疫苗的免疫原性,上述诸多因素导致流感疫苗的生产效率和有效性大打折扣。mRNA技术有望从根本上克服上述缺陷,为研发下一代流感疫苗提供新手段。自2012年首次证实流感病毒mRNA疫苗可在动物体内诱导产生保护性免疫应答以来[77],研究者评价了多种递送系统和核酸修饰手段对此类疫苗免疫保护效果的影响。在流感病毒mRNA疫苗研究领域,Moderna公司研发的甲型H7N9 (mRNA-1440)和甲型H10N8 (mRNA-1851)流感病毒mRNA疫苗编码全长的血凝素蛋白,于2016年率先进入一期临床试验,并显示出良好的免疫原性和耐受性[78]。此外,mRNA技术在通用型流感疫苗的研发中也进行了尝试,Pardi等设计了编码甲型H1N1流感病毒Cal09株血凝素蛋白茎部保守区的mRNA疫苗,经LNP递送至小鼠、雪貂和家兔体内后均能诱导针对该保守区的特异抗体,特别是能同时保护小鼠免受甲型H1N1 PR8株和禽流感病毒H5N1的致死性感染[79],展现出了良好的应用前景。研究者将进一步结合传统流感疫苗的研究发现,充分利用mRNA疫苗设计简便快速的优点,不断调整优化配伍抗原序列,进而研发获得具有更好免疫保护效果的流感疫苗。
2.4 其他病毒mRNA疫苗mRNA技术在其他病毒传染病疫苗研发中也取得了重要进展,巨细胞病毒、呼吸道合胞病毒、狂犬病毒、基孔肯雅病毒等mRNA疫苗均进入临床试验阶段[33]。埃博拉病毒和HIV的mRNA疫苗的临床前研究也取得了令人振奋的结果,如将埃博拉病毒保护性抗原糖蛋白基因插入到委内瑞拉马脑炎病毒复制子,进一步包裹进树状多聚物纳米颗粒后免疫小鼠,小鼠能产生明显的糖蛋白特异的IgG抗体、IFN-γ和IL-2,并能有效对抗致死剂量埃博拉病毒的感染[80]。另一种编码糖蛋白并由LNP递送的修饰型埃博拉病毒mRNA疫苗也能保护豚鼠免受致死剂量病毒的攻击[81]。研究者也尝试采用阳离子纳米乳液、聚合物纳米颗粒以及LNP递送HIV的mRNA疫苗,积累了丰富的免疫应答数据,为后续抗原序列和递送方式的优化及临床研究奠定了重要基础[82-84]。
2.5 mRNA抗体mRNA抗体技术的概念验证于2017年由mRNA技术先驱Weissman的研究团队最先完成,研究人员将经过m1ψ修饰的编码HIV广谱中和抗体VRC01的轻链和重链基因的mRNA以1:1混合,包裹于LNP后经静脉注释至小鼠体内,24 h后血清中的抗体滴度即达峰值,并能稳定表达5 d,同时能为小鼠提供有效的免疫保护[85]。Kose等开发了基孔肯雅病毒中和单抗CHKV-24的mRNA抗体,以0.5 mg/kg剂量静脉给药可为小鼠提供完全的保护,也能有效缓解猴体感染病毒后出现的多发性关节炎[49]。该抗体也是第一个进入一期临床试验阶段的mRNA抗体(临床试验名称为mRNA-1944),期中数据显示出良好的耐受性,在静脉给药后24 h血清中的抗体水平即可达峰值,半衰期为2个月,为下一步开展更大规模的临床研究奠定了良好基础[86]。新型冠状病毒mRNA抗体也有重要进展,Li等利用甲病毒复制子颗粒系统成功构建获得了自我复制型的mRNA抗体VEEV-VRP-CB6,并通过滴鼻途径递送至小鼠肺部,有效降低了小鼠肺部和气管中的病毒载量[87]。该策略表明,甲病毒复制子系统也可成为一种新的mRNA疫苗递送方式,有望解决LNP滴鼻免疫效果不佳的瓶颈,为滴鼻型mRNA疫苗的设计和应用提供了新的思路。
3 展望新型冠状病毒全球大流行是21世纪人类遭遇的第一次也是最严重的全球性瘟疫,而新型冠状病毒mRNA疫苗以明显优势从众多疫苗中脱颖而出,标志着mRNA技术理念在经历三十多年不懈的科学探索后正式落地。与此同时,以BioNTech、CureVac和Moderna为代表的国际mRNA技术企业已布局了艾滋病、流感、埃博拉、寨卡病毒等重要新老传染病的mRNA疫苗研发管线,多个疫苗已进入临床一期或二期试验。特别是在公认最难攻克的HIV疫苗领域,Moderna公司研发的mRNA疫苗已于2021年8月启动临床一期试验,其能否成功将成为验证mRNA疫苗有效性的又一项重要尝试。我国的mRNA技术企业在抗击新型冠状病毒疫情中迎难而上,多款新型冠状病毒mRNA疫苗先后进入临床研究,上述企业也在积极布局疫病疫苗和肿瘤治疗等产品管线,以期在未来mRNA技术领域的国际竞争中占据主动。
mRNA技术彻底更新了人们对疫苗的认识,但该领域仍有诸多亟须解决的问题。首先是如何使外源mRNA更有效逃避人体天然免疫系统的识别,进而降低或消除由此引起的发热和炎症等不良反应,同时也需降低递送载体组分免疫原性引发的负面效应,进一步提高疫苗的安全性;其次是如何使mRNA刺激产生更真实、全面的保护性免疫应答,提高有效性的同时降低接种剂量,进而间接提升mRNA产能;最后是解决mRNA制剂需超低温保藏和冷链运输等难题,降低mRNA制剂对保藏和运输条件的超高要求,为疫苗和抗体在经济欠发达地区的使用铺平道路。解决上述问题一方面依赖于技术方法和材料的革新,需要生物学、化学、材料学、物理学等多学科的新技术理念的充分交叉融合,研发可逃避天然免疫系统识别的mRNA序列设计与修饰新技术,开发靶向可控的智能核酸递送和释放新方法,研制人体更耐受以及耐高温的mRNA递送和储存运输新材料;另一方面也取决于我们理解免疫保护机制的广度和深度,这需要包括微生物学、免疫学、计算生物学等领域的科学工作者通力合作,将各领域对生命本质的不同理解有机融合,全面认识保护性免疫应答如何发生发展这一根本问题,从而为mRNA疫苗和抗体的设计开发提供全景式的科学理论支撑。新型冠状病毒mRNA疫苗的成功研发不仅为人类未来战胜新老传染病带来光明愿景,整个生物医学领域也将迎来革命性突破,值得我们持续关注。
| [1] |
Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo[J]. Science, 1990, 247(4949 Pt 1): 1465-1468. |
| [2] |
Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA[J]. Science, 1992, 255(5047): 996-998. DOI:10.1126/science.1546298 |
| [3] |
Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective[J]. Npj Vaccines, 2020, 5: 11. DOI:10.1038/s41541-020-0159-8 |
| [4] |
Blakney AK, Ip S, Geall AJ. An update on self-amplifying mRNA vaccine development[J]. Vaccines, 2021, 9(2): 97. DOI:10.3390/vaccines9020097 |
| [5] |
Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines[J]. Advanced Drug Delivery Reviews, 2021, 170: 83-112. DOI:10.1016/j.addr.2020.12.014 |
| [6] |
Kallen KJ, Heidenreich R, Schnee M, Petsch B, Schlake T, Thess A, Baumhof P, Scheel B, Koch SD, Fotin-Mleczek M. A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines[J]. Human Vaccines & Immunotherapeutics, 2013, 9(10): 2263-2276. |
| [7] |
Sullenger BA, Nair S. From the RNA world to the clinic[J]. Science, 2016, 352(6292): 1417-1420. DOI:10.1126/science.aad8709 |
| [8] |
Ulmer JB, Geall AJ. Recent innovations in mRNA vaccines[J]. Current Opinion in Immunology, 2016, 41: 18-22. DOI:10.1016/j.coi.2016.05.008 |
| [9] |
Kumar P, Sweeney TR, Skabkin MA, Skabkina OV, Hellen CUT, Pestova TV. Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5′-terminal regions of cap0-, cap1- and 5′ppp- mRNAs[J]. Nucleic Acids Research, 2013, 42(5): 3228-3245. |
| [10] |
Jang SK, Paek KY. Cap-dependent translation is mediated by 'RNA looping' rather than 'ribosome scanning'[J]. RNA Biology, 2016, 13(1): 1-5. DOI:10.1080/15476286.2015.1107700 |
| [11] |
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines: a new era in vaccinology[J]. Nature Reviews Drug Discovery, 2018, 17(4): 261-279. DOI:10.1038/nrd.2017.243 |
| [12] |
Jemielity J, Fowler T, Zuberek J, Stepinski J, Lewdorowicz M, Niedzwiecka A, Stolarski R, Darzynkiewicz E, Rhoads RE. Novel "anti-reverse" cap analogs with superior translational properties[J]. RNA, 2003, 9(9): 1108-1122. DOI:10.1261/rna.5430403 |
| [13] |
Pascolo S. Synthetic messenger RNA-based vaccines: from scorn to hype[J]. Viruses, 2021, 13(2): 270. DOI:10.3390/v13020270 |
| [14] |
Henderson JM, Ujita A, Hill E, Yousif-Rosales S, Smith C, Ko N, McReynolds T, Cabral CR, Escamilla-Powers JR, Houston ME. Correction: cap 1 messenger RNA synthesis with co-transcriptional CleanCap® analog by in vitro transcription[J]. Current Protocols, 2021, 1(12): e336. |
| [15] |
Asrani KH, Farelli JD, Stahley MR, Miller RL, Cheng CJ, Subramanian RR, Brown JM. Optimization of mRNA untranslated regions for improved expression of therapeutic mRNA[J]. RNA Biology, 2018, 15(6): 756-762. |
| [16] |
Orlandini Von Niessen AG, Poleganov MA, Rechner C, Plaschke A, Kranz LM, Fesser S, Diken M, Löwer M, Vallazza B, Beissert T, et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening[J]. Molecular Therapy, 2019, 27(4): 824-836. DOI:10.1016/j.ymthe.2018.12.011 |
| [17] |
Sample PJ, Wang B, Reid DW, Presnyak V, McFadyen IJ, Morris DR, Seelig G. Human 5′ UTR design and variant effect prediction from a massively parallel translation assay[J]. Nature Biotechnology, 2019, 37(7): 803-809. DOI:10.1038/s41587-019-0164-5 |
| [18] |
Jain R, Frederick JP, Huang EY, Burke KE, Mauger DM, Andrianova EA, Farlow SJ, Siddiqui S, Pimentel J, Cheung-Ong K, et al. microRNAs enable mRNA therapeutics to selectively program cancer cells to self-destruct[J]. Nucleic Acid Therapeutics, 2018, 28(5): 285-296. DOI:10.1089/nat.2018.0734 |
| [19] |
Hewitt SL, Bai AL, Bailey D, Ichikawa K, Zielinski J, Karp R, Apte A, Arnold K, Zacharek SJ, Iliou MS, et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs[J]. Science Translational Medicine, 2019, 11(477): eaat9143. DOI:10.1126/scitranslmed.aat9143 |
| [20] |
Leppek K, Das R, Barna M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them[J]. Nature Reviews Molecular Cell Biology, 2018, 19(3): 158-174. DOI:10.1038/nrm.2017.103 |
| [21] |
Schlake T, Thess A, Thran M, Jordan I. mRNA as novel technology for passive immunotherapy[J]. Cellular and Molecular Life Sciences: CMLS, 2019, 76(2): 301-328. DOI:10.1007/s00018-018-2935-4 |
| [22] |
Thess A, Grund S, Mui BL, Hope MJ, Baumhof P, Fotin-Mleczek M, Schlake T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals[J]. Molecular Therapy: the Journal of the American Society of Gene Therapy, 2015, 23(9): 1456-1464. DOI:10.1038/mt.2015.103 |
| [23] |
Kudla G, Lipinski L, Caffin F, Helwak A, Zylicz M. High guanine and cytosine content increases mRNA levels in mammalian cells[J]. PLoS Biology, 2006, 4(6): e180. DOI:10.1371/journal.pbio.0040180 |
| [24] |
Wayment-Steele HK, Kim DS, Choe CA, Nicol JJ, Wellington-Oguri R, Watkins AM, Parra Sperberg RA, Huang PS, Participants E, Das R. Theoretical basis for stabilizing messenger RNA through secondary structure design[J]. Nucleic Acids Research, 2021, 49(18): 10604-10617. DOI:10.1093/nar/gkab764 |
| [25] |
Lima SA, Chipman LB, Nicholson AL, Chen YH, Yee BA, Yeo GW, Coller J, Pasquinelli AE. Short poly(A) tails are a conserved feature of highly expressed genes[J]. Nature Structural & Molecular Biology, 2017, 24(12): 1057-1063. |
| [26] |
Grier AE, Burleigh S, Sahni J, Clough CA, Cardot V, Choe DC, Krutein MC, Rawlings DJ, Jensen MC, Scharenberg AM, et al. pEVL: a linear plasmid for generating mRNA IVT templates with extended encoded poly(A) sequences[J]. Molecular Therapy-Nucleic Acids, 2016, 5: e306. DOI:10.1038/mtna.2016.21 |
| [27] |
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine[J]. The New England Journal of Medicine, 2021, 384(5): 403-416. DOI:10.1056/NEJMoa2035389 |
| [28] |
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine[J]. Annals of Internal Medicine, 2020, 383(27): 2603-2615. |
| [29] |
Wang HM, Hu X, Huang MY, Liu J, Gu Y, Ma LJ, Zhou Q, Cao XT. Mettl3-mediated mRNA m6A methylation promotes dendritic cell activation[J]. Nature Communications, 2019, 10: 1898. DOI:10.1038/s41467-019-09903-6 |
| [30] |
Chen RY, Zhang H, Yan JX, Bryers JD. Scaffold-mediated delivery for non-viral mRNA vaccines[J]. Gene Therapy, 2018, 25(8): 556-567. DOI:10.1038/s41434-018-0040-9 |
| [31] |
Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies[J]. European Journal of Immunology, 2000, 30(1): 1-7. DOI:10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-# |
| [32] |
Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness[J]. Nature, 2020, 586(7830): 567-571. DOI:10.1038/s41586-020-2622-0 |
| [33] |
Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation[J]. Nature Reviews Drug Discovery, 2021, 20(11): 817-838. DOI:10.1038/s41573-021-00283-5 |
| [34] |
La Manna P, De Rosa M, Talotta C, Rescifina A, Floresta G, Soriente A, Gaeta C, Neri P. Synergic interplay between halogen bonding and hydrogen bonding in the activation of a neutral substrate in a nanoconfined space[J]. Angewandte Chemie: International Ed in English, 2020, 59(2): 811-818. DOI:10.1002/anie.201909865 |
| [35] |
Miao L, Li LX, Huang YX, Delcassian D, Chahal J, Han JS, Shi YH, Sadtler K, Gao WT, Lin JQ, et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation[J]. Nature Biotechnology, 2019, 37(10): 1174-1185. DOI:10.1038/s41587-019-0247-3 |
| [36] |
Ke XY, Shelton L, Hu YZ, Zhu YN, Chow E, Tang HY, Santos JL, Mao HQ. Surface-functionalized PEGylated nanoparticles deliver messenger RNA to pulmonary immune cells[J]. ACS Applied Materials & Interfaces, 2020, 12(32): 35835-35844. |
| [37] |
Tan L, Zheng T, Li M, Zhong XF, Tang Y, Qin M, Sun X. Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates[J]. Drug Delivery and Translational Research, 2020, 10(3): 678-689. DOI:10.1007/s13346-020-00725-4 |
| [38] |
Kaczmarek JC, Kauffman KJ, Fenton OS, Sadtler K, Patel AK, Heartlein MW, DeRosa F, Anderson DG. Optimization of a degradable polymer-lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells[J]. Nano Letters, 2018, 18(10): 6449-6454. DOI:10.1021/acs.nanolett.8b02917 |
| [39] |
Kaczmarek JC, Patel AK, Kauffman KJ, Fenton OS, Webber MJ, Heartlein MW, DeRosa F, Anderson DG. Polymer-lipid nanoparticles for systemic delivery of mRNA to the lungs[J]. Angewandte Chemie: International Ed in English, 2016, 55(44): 13808-13812. DOI:10.1002/anie.201608450 |
| [40] |
Kim HJ, Ogura S, Otabe T, Kamegawa R, Sato M, Kataoka K, Miyata K. Fine-tuning of hydrophobicity in amphiphilic polyaspartamide derivatives for rapid and transient expression of messenger RNA directed toward genome engineering in brain[J]. ACS Central Science, 2019, 5(11): 1866-1875. DOI:10.1021/acscentsci.9b00843 |
| [41] |
McKinlay CJ, Vargas JR, Blake TR, Hardy JW, Kanada M, Contag CH, Wender PA, Waymouth RM. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals[J]. PNAS, 2017, 114(4): E448-E456. |
| [42] |
Haabeth OAW, Blake TR, McKinlay CJ, Waymouth RM, Wender PA, Levy R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice[J]. PNAS, 2018, 115(39): E9153-E9161. |
| [43] |
Nissim A, Chernajovsky Y. Historical development of monoclonal antibody therapeutics[J]. Handbook of Experimental Pharmacology, 2008(181): 3-18. |
| [44] |
Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market[J]. mAbs, 2015, 7(1): 9-14. DOI:10.4161/19420862.2015.989042 |
| [45] |
Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020[J]. mAbs, 2020, 12(1): 1703531. DOI:10.1080/19420862.2019.1703531 |
| [46] |
Sabnis S, Kumarasinghe ES, Salerno T, Mihai C, Ketova T, Senn JJ, Lynn A, Bulychev A, McFadyen I, Chan J, et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates[J]. Molecular Therapy: the Journal of the American Society of Gene Therapy, 2018, 26(6): 1509-1519. DOI:10.1016/j.ymthe.2018.03.010 |
| [47] |
Khoshnejad M, Patel A, Wojtak K, Kudchodkar SB, Humeau L, Lyssenko NN, Rader DJ, Muthumani K, Weiner DB. Development of novel DNA-encoded PCSK9 monoclonal antibodies as lipid-lowering therapeutics[J]. Molecular Therapy, 2019, 27(1): 188-199. DOI:10.1016/j.ymthe.2018.10.016 |
| [48] |
Balazs AB, Chen J, Hong CM, Rao DS, Yang LL, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis[J]. Nature, 2012, 481(7379): 81-84. DOI:10.1038/nature10660 |
| [49] |
Kose N, Fox JM, Sapparapu G, Bombardi R, Tennekoon RN, De Silva AD, Elbashir SM, Theisen MA, Humphris-Narayanan E, Ciaramella G, et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection[J]. Science Immunology, 2019, 4(35): eaaw6647. DOI:10.1126/sciimmunol.aaw6647 |
| [50] |
Deal CE, Carfi A, Plante OJ. Advancements in mRNA encoded antibodies for passive immunotherapy[J]. Vaccines, 2021, 9(2): 108. DOI:10.3390/vaccines9020108 |
| [51] |
Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, et al. Phase Ⅰ/Ⅱ study of COVID-19 RNA vaccine BNT162b1 in adults[J]. Nature, 2020, 586(7830): 589-593. DOI:10.1038/s41586-020-2639-4 |
| [52] |
Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates[J]. The New England Journal of Medicine, 2020, 383(25): 2439-2450. DOI:10.1056/NEJMoa2027906 |
| [53] |
Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses[J]. Nature, 2020, 586(7830): 594-599. DOI:10.1038/s41586-020-2814-7 |
| [54] |
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting[J]. The New England Journal of Medicine, 2021, 384(15): 1412-1423. DOI:10.1056/NEJMoa2101765 |
| [55] |
Martin C, Lowery D. mRNA vaccines: intellectual property landscape[J]. Nature Reviews Drug Discovery, 2020, 19(9): 578. DOI:10.1038/d41573-020-00119-8 |
| [56] |
Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, Flach B, O'Connell S, Bock KW, Minai M, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates[J]. The New England Journal of Medicine, 2020, 383(16): 1544-1555. DOI:10.1056/NEJMoa2024671 |
| [57] |
Zhang NN, Li XF, Deng YQ, Zhao H, Huang YJ, Yang G, Huang WJ, Gao P, Zhou C, Zhang RR, et al. A thermostable mRNA vaccine against COVID-19[J]. Cell, 2020, 182(5): 1271-1283.e16. DOI:10.1016/j.cell.2020.07.024 |
| [58] |
Chen GL, Li XF, Dai XH, Li N, Cheng ML, Huang Z, Shen J, Ge YH, Shen ZW, Deng YQ, et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomized, double-blind, placebo-controlled, phase 1 trial[J]. The Lancet Microbe, 2022, 3(3): e193-e202. DOI:10.1016/S2666-5247(21)00280-9 |
| [59] |
Pozzetto B, Legros V, Djebali S, Barateau V, Guibert N, Villard M, Peyrot L, Allatif O, Fassier JB, Massardier-Pilonchéry A, et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination[J]. Nature, 2021, 600(7890): 701-706. DOI:10.1038/s41586-021-04120-y |
| [60] |
Normark J, Vikström L, Gwon YD, Persson IL, Edin A, Björsell T, Dernstedt A, Christ W, Tevell S, Evander M, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination[J]. The New England Journal of Medicine, 2021, 385(11): 1049-1051. DOI:10.1056/NEJMc2110716 |
| [61] |
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of covid-19 vaccines against the B. 1.617.2 (delta) variant[J]. Missouri Medicine, 2021, 385(7): 585-594. |
| [62] |
Lucas C, Vogels CBF, Yildirim I, Rothman JE, Lu PW, Monteiro V, Gehlhausen JR, Campbell M, Silva J, Tabachnikova A, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity[J]. Nature, 2021, 600(7889): 523-529. DOI:10.1038/s41586-021-04085-y |
| [63] |
Neidleman J, Luo XY, McGregor M, Xie GR, Murray V, Greene WC, Lee SA, Roan NR. mRNA vaccine-induced T cells respond identically to SARS-CoV-2 variants of concern but differ in longevity and homing properties depending on prior infection status[J]. eLife, 2021, 10: e72619. DOI:10.7554/eLife.72619 |
| [64] |
Amanat F, Thapa M, Lei T, Ahmed SMS, Adelsberg DC, Carreño JM, Strohmeier S, Schmitz AJ, Zafar S, Zhou JQ, et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2[J]. Cell, 2021, 184(15): 3936-3948.e10. DOI:10.1016/j.cell.2021.06.005 |
| [65] |
Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, Baxter AE, Herati RS, Oldridge DA, Gouma S, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination[J]. Immunity, 2021, 54(9): 2133-2142.e3. DOI:10.1016/j.immuni.2021.08.001 |
| [66] |
Ciabattini A, Pastore G, Fiorino F, Polvere J, Lucchesi S, Pettini E, Auddino S, Rancan I, Durante M, Miscia M, et al. Evidence of SARS-CoV-2-specific memory B cells six months after vaccination with the BNT162b2 mRNA vaccine[J]. Frontiers in Immunology, 2021, 12: 740708. DOI:10.3389/fimmu.2021.740708 |
| [67] |
Woldemeskel BA, Garliss CC, Blankson JN. mRNA vaccine-elicited SARS-CoV-2-specific T cells persist at 6 months and recognize the delta variant[J]. Clinical Infectious Diseases, 2021. |
| [68] |
Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, Donahue JG, Kharbanda EO, Naleway A, Nelson JC, et al. Surveillance for adverse events after COVID-19 mRNA vaccination[J]. JAMA, 2021, 326(14): 1390-1399. DOI:10.1001/jama.2021.15072 |
| [69] |
Sun H. On preliminary findings of mRNA covid-19 vaccine safety in pregnant persons[J]. The New England Journal of Medicine, 2021, 385(16): 1535-1536. DOI:10.1056/NEJMc2113516 |
| [70] |
Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel[J]. The New England Journal of Medicine, 2021, 385(23): 2140-2149. DOI:10.1056/NEJMoa2109730 |
| [71] |
Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, et al. Myocarditis after COVID-19 vaccination in a large health care organization[J]. The New England Journal of Medicine, 2021, 385(23): 2132-2139. DOI:10.1056/NEJMoa2110737 |
| [72] |
Rodríguez C, Pérez-Nieva A, Máiz L, Meijón M, Llamas P, Monreal M, Bikdeli B, Jiménez D. Vaccine-induced immune thrombotic thrombocytopenia after the BNT162b2 mRNA COVID-19 vaccine: a case study[J]. Thrombosis Research, 2021, 208: 1-3. DOI:10.1016/j.thromres.2021.10.002 |
| [73] |
Liu ZY, Shi WF, Qin CF. The evolution of Zika virus from Asia to the Americas[J]. Nature Reviews Microbiology, 2019, 17(3): 131-139. DOI:10.1038/s41579-018-0134-9 |
| [74] |
Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination[J]. Nature, 2017, 543(7644): 248-251. DOI:10.1038/nature21428 |
| [75] |
Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. Modified mRNA vaccines protect against zika virus infection[J]. Cell, 2017, 168(6): 1114-1125.e10. DOI:10.1016/j.cell.2017.02.017 |
| [76] |
Faria NR, Da Silva Azevedo RDS, Kraemer MUG, Souza R, Cunha MS, Hill SC, Thézé J, Bonsall MB, Bowden TA, Rissanen I, et al. Zika virus in the Americas: early epidemiological and genetic findings[J]. Science, 2016, 352(6283): 345-349. DOI:10.1126/science.aaf5036 |
| [77] |
Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, Schlake T, Thess A, Kallen KJ, Stitz L, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection[J]. Nature Biotechnology, 2012, 30(12): 1210-1216. DOI:10.1038/nbt.2436 |
| [78] |
Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses[J]. Molecular Therapy, 2017, 25(6): 1316-1327. DOI:10.1016/j.ymthe.2017.03.035 |
| [79] |
Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, Tam YK, Karikó K, Barbosa CJ, Madden TD, et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies[J]. Nature Communications, 2018, 9: 3361. DOI:10.1038/s41467-018-05482-0 |
| [80] |
Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, Sidik SM, Lourido S, Langer R, Bavari S, et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose[J]. PNAS, 2016, 113(29): E4133-E4142. |
| [81] |
Meyer M, Huang E, Yuzhakov O, Ramanathan P, Ciaramella G, Bukreyev A. Modified mRNA-based vaccines elicit robust immune responses and protect Guinea pigs from Ebola virus disease[J]. The Journal of Infectious Diseases, 2018, 217(3): 451-455. DOI:10.1093/infdis/jix592 |
| [82] |
Brito LA, Chan M, Shaw CA, Hekele A, Carsillo T, Schaefer M, Archer J, Seubert A, Otten GR, Beard CW, et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines[J]. Molecular Therapy, 2014, 22(12): 2118-2129. DOI:10.1038/mt.2014.133 |
| [83] |
Zhao MN, Li M, Zhang ZR, Gong T, Sun X. Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA[J]. Drug Delivery, 2016, 23(7): 2596-2607. DOI:10.3109/10717544.2015.1038856 |
| [84] |
Pardi N, LaBranche CC, Ferrari G, Cain DW, Tombácz I, Parks RJ, Muramatsu H, Mui BL, Tam YK, Karikó K, et al. Characterization of HIV-1 nucleoside-modified mRNA vaccines in rabbits and rhesus macaques[J]. Molecular Therapy - Nucleic Acids, 2019, 15: 36-47. DOI:10.1016/j.omtn.2019.03.003 |
| [85] |
Pardi N, Secreto AJ, Shan XC, Debonera F, Glover J, Yi YJ, Muramatsu H, Ni HP, Mui BL, Tam YK, et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge[J]. Nature Communications, 2017, 8: 14630. DOI:10.1038/ncomms14630 |
| [86] |
Fulton BO, Sachs D, Schwarz MC, Palese P, Evans MJ. Transposon mutagenesis of the Zika virus genome highlights regions essential for RNA replication and restricted for immune evasion[J]. Journal of Virology, 2017, 91(15): e00698-e00617. |
| [87] |
Li JQ, Zhang ZR, Zhang HQ, Zhang YN, Zeng XY, Zhang QY, Deng CL, Li XD, Zhang B, Ye HQ. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice[J]. Signal Transduction and Targeted Therapy, 2021, 6: 369. DOI:10.1038/s41392-021-00783-1 |
 2022, Vol. 49
2022, Vol. 49