扩展功能
文章信息
- 吴盛露, 杜海, 徐岩
- WU Shenglu, DU Hai, XU Yan
- 生物热对传统固态发酵菌群演替及其代谢影响的研究进展
- Effect of bio-heat on microbial succession and metabolism in traditional solid-state fermentation: a review
- 微生物学通报, 2022, 49(6): 2281-2294
- Microbiology China, 2022, 49(6): 2281-2294
- DOI: 10.13344/j.microbiol.china.210924
-
文章历史
- 收稿日期: 2021-10-08
- 接受日期: 2022-02-12
- 网络首发日期: 2022-03-22
2. 江南大学工业生物技术教育部重点实验室,江苏 无锡 214122
2. Key Laboratory of Industrial Biotechnology, Ministry of Education, Jiangnan University, Wuxi 214122, Jiangsu, China
固态发酵(solid-state fermentation,SSF)是指微生物在没有或几乎没有自由流动水的天然固态培养基质或浸渍惰性载体上进行的发酵方式[1]。固态发酵最早源于食物的保存,多用于传统发酵食品领域,如奶酪、酿酒、制酱、豆豉等。传统固态发酵通过富集环境中的微生物进行开放式发酵,是一种由微生物自发驱动群体代谢过程的复杂发酵体系,具有多菌共存、多酶协同和多途径代谢等特征[2-3]。以白酒为例,白酒酿造体系富集了来源于自然环境、原料与种曲的多种微生物,如乳酸菌属(Lactobacillus)、芽孢杆菌属(Bacillus)、酵母菌属(Saccharomyces)及曲霉属(Aspergillus),发酵菌群内部相互制衡,共同维持微生态下的稳态[4]。
微生物利用淀粉、纤维素、脂肪和蛋白质等其他营养物质进行生长繁殖与代谢,过程中会产生能量,除了以高能化合物的形式(如ATP)积累于细胞中以供微生物细胞合成和代谢需要外,其余以热的形式散发出来,即为生物热。这种由微生物代谢自发产生的热量在传热性差的固态基质中逐步积累形成了典型的温度效应,显著影响微生物菌群的多样性与代谢功能[5-6]。例如,一些发酵体系中(如大曲、堆肥等)由于热量积聚导致发酵温度可高达50 ℃以上[3, 7]。有研究指出,微生物群落中嗜热与耐热性微生物的存在保证了高温胁迫环境下群落功能[8-10]。随着微生物组学与分子生物学的发展,研究者们逐步明确了发酵微生物种类与丰度、演替规律、代谢功能等,然而关于自发富集的微生物群落结构与功能如何随着温度变化而发生演替,以及微生物菌群如何适应高温的潜在机理仍不清楚。解析生物热对群体微生物的影响,了解高温环境下的微生物代谢特征是实现固态发酵可控的重要环节。因此,本文主要从传统固态发酵方面阐述微生物在菌群结构、代谢途径和功能基因水平等方面受生物热胁迫的影响及耐热机制,解读微生物菌群对高温胁迫的调控与适应,以期为高温发酵的机制阐明及工艺优化提供理论依据。
1 传统固态发酵与生物热传统固态发酵的识别本质在于固体基质,不仅为微生物生长和代谢提供碳和能量来源,还与气体、液体组成三相共存的微环境体系。传统固态发酵前期,适宜的环境与营养条件促使发酵体系中微生物菌群的快速繁殖和代谢。微生物利用固体营养物进行生理活动时会分泌多种降解酶,以及一些低聚糖、乙醇、氨基酸、小肽、游离脂肪酸等代谢产物。除了这些之外,这种快速发酵还伴随着生物热的不断释放。
固态基质中传热困难所导致的生物热积聚,在无法均匀搅拌的情况下进而引发发酵基质的局部高温是传统固态发酵的典型特征。热量的积累主要由产热和散热二者决定。产热主要受微生物生长代谢的影响,与其代谢活性强度密切相关,受到微生物含量、种类、环境因子(温度、pH、氧气等)的影响[11]。散热与固态基质中三相共存的特性及气体和固体的导热性能有关[7]。在固态发酵过程中,固态基质一般采用导热系数低的农作物或农工业废弃物。此外,微生物在固态基质上的生长与代谢使得固态基质中气、液、固的质量比或体积比发生变化,甚至物质的种类发生了变化,从而间接引起固态基质的总传热系数发生变化[7, 12-13]。因而在微生物持续降解底物过程中,热量逐渐在材料中集聚,使物料的温度升高,从而影响微生物的生长及发酵产物的代谢。在大规模的固态发酵中,为防止发酵温度过高影响微生物发酵,往往采取提高曝气量和翻拌频率作为主要的散热方式。
尽管固态发酵过程相对较复杂,但也具有明显的规律性。为了探索白酒固态发酵工艺,Jin等[12]利用Han-Levenspiel方程联合多种发酵参数建立了发酵地缸内物质传递及能量传递模型,并通过实验数据中核心产物(酒精、乳酸)的变化验证了微生物进行酒精发酵是厌氧固态发酵中产热的核心,而缸内径向传导是最重要的传热机制(占去除热量的79%),水蒸发有重要的影响(16%),垂直传导几乎可以忽略不计(4%)。Zhang等[13]构建了用于表征介质物理性质的力学指标mechanical property index (Imp),进而探讨固体介质物理特性与发酵性能的关系,结果表明在发酵的初始阶段,Imp≤4.37×10−2时与热传导和保水性正相关,有利于细胞生长;Imp > 4.37×10−2时,发酵过程由于热量积聚及较差的渗透性进而降低发酵性能。可见固态介质的物理性质与传导热量的形式均会显著影响发酵过程的传热效果,进而影响发酵性能。Zhang等[6]研究起始发酵温度对固态发酵的影响,发现较高的起始温度改变了微生物多样性,加速了乳酸菌数量增长,使微生物的演替速率加快,也提高了酸、酯的含量。Wang等[14]发现日平均气温是固态发酵中的微生物菌群与代谢产物变化的主要决定因素,并且季节性的变化显著影响传统发酵体系,导致代谢功能和代谢产物的差异,从而威胁到发酵产品质量的稳定性。发酵体系内或是环境温度均会影响固态发酵中的微生物多样性和时空演替规律,甚至是代谢产物种类、含量等,表明控制温度是维持传统固态稳定发酵的关键之一。因此,解析不同温度控制下微生物的代谢特征并基于温度靶向调控微生物群落与代谢是宏观调控固态发酵的必然趋势。
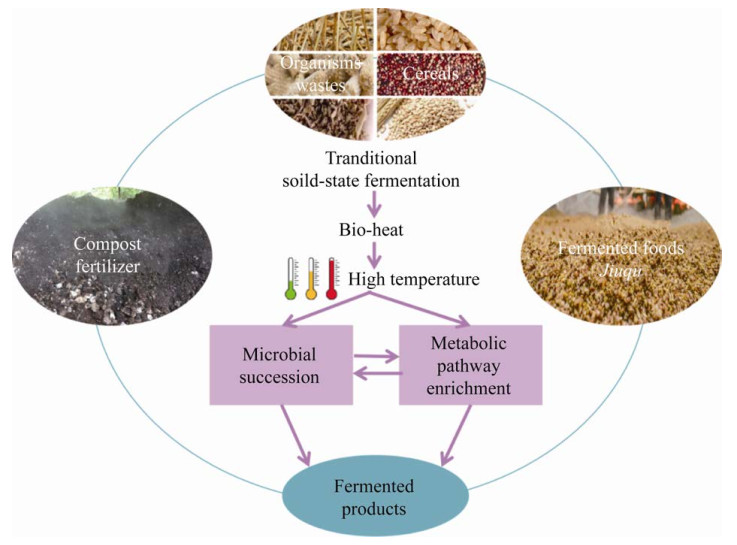
2 生物热介导的高温对菌群的胁迫作用生物热介导的高温影响发酵过程中复杂的微生物群落,宏观上表现为群落的演替,微观上表现为代谢通路、功能基因的差异化富集(图 1)。目前对于发酵体系的研究已从单一分离菌株或混合培养转到多角度分析高度多样性的原位微生物,系统解析复杂群落的发酵机制[15-17]。用于研究微生物群落的方式主要包括早期的PCR-DGGE、T-RFLP等,以及高通量测序技术如扩增子测序、宏基因组、宏转录组和宏蛋白组[18]。
|
| 图 1 传统高温固态发酵类型 Figure 1 The types of the traditional high temperature solid-state fermentation types. |
|
|
固态发酵过程中温度与微生物群落组成和种群的演替具有显著相关性:初期温度适应大部分微生物生长,造成温度稳定上升,初期微生物的代谢分解为发酵环境提供可用的营养因子;进入高温阶段,耐热性强或嗜热的微生物取代部分中温微生物继续生长,温度持续升高,直到温度达到顶温,这时除了耐热性更强的孢子外大多数微生物死亡或休眠;随后温度开始下降[19]。此外,温度也被作为反映发酵效率最直接、最敏感的变量之一,可被用于筛选微生物以获取高效的有机物转化效能[20]。
白酒发酵剂(大曲)作为白酒生产中最重要的组成部分,具有糖化、发酵和生香等功能。根据制曲中达到的顶温,可将大曲分为低温大曲(清香曲,40−50 ℃)、中温大曲(浓香曲,50−60 ℃)和高温大曲(酱香曲,60−70 ℃)。这3种类型大曲的微生物群落存在较大差异,其中高温大曲中主要由大量嗜热、耐热的微生物组成,如芽孢杆菌、丝状真菌等[21]。方程等[22]通过荧光定量PCR对高温曲的丝状真菌进行过程追踪,发现丝状真菌的总生物量随着大曲温度的升高而下降,但嗜热真菌Thermomyces的丰度随大曲温度的升高而上升,表明温度的升高能够富集嗜热性状的微生物菌株。Xiao等[19]在研究中温大曲微生物群落结构变异的过程中发现,前期细菌和真菌的快速繁殖导致发酵温度快速达到55 ℃,而这种由生物热介导的高温在中后期明显影响了大多数微生物的生长,只剩下耐热类群存活或继续生长,例如Thermomyces、Bacillus、高温放线菌属(Thermoactinomyces)及嗜热子囊菌属(Thermoascus)。固态发酵过程中由生物热积累的高温会反作用于菌群结构,造成微生物群落结构的变异,使其具有耐热特征。
堆肥可将有机废料分解成稳定、可用的有机物质。连续高温堆肥、超高温堆肥等技术更有助于有机物的快速降解和成熟。Yu等[23]通过比较传统堆肥与超高温堆肥过程发现,微生物群落结构的差异是引起发酵环境温度差异的重要原因;其中,在超高温堆肥过程中80 ℃高温条件有利于嗜热细菌[如Thermaceae 35.5%−53.3%、高温放线菌科(Thermoactinomycetaceae) 4.0%−30.0%]的生长,进而提高有机肥料的降解效率。Zhang等[24]利用多元回归树对堆肥发酵过程中微生物群落结构分析发现,细菌和真菌群落存在不同的时间演替规律,细菌群落对温度变化的响应比真菌群落更敏感;他们基于随机森林分析建立了堆肥微生物群落组成与堆肥周期的关联模型挖掘堆肥过程中特定阶段的标志微生物,结果表明链孢囊菌科(Streptosporangiaceae)、特吕珀菌科(Trueperaceae)和小梨菌科(Pirellulaceae)是细菌的标志微生物,而酵母科(Saccharomycetales)和毛壳菌科(Chaetomiaceae)是真菌的标志微生物。通过对不同阶段差异菌株的挖掘,有助于靶向寻找高温环境下的关键代谢菌株,并理解温度驱动下的群落微生态。
2.2 高温富集菌群代谢通路与功能基因在大曲研究中,Yi等[25]利用转录组学方法鉴定出高温大曲中Aspergillus、Penicillium等真菌可能是高温阶段最具活性的微生物成员,还发现淀粉和蔗糖代谢、糖酵解、丙酮酸代谢和柠檬酸循环是关键的碳水化合物和能量代谢途径,这些途径中产生的中间产物对乙醇和风味的生成是必需的。此外,Huang等[26]通过细菌群落功能预测发现,高温大曲富集更高丰度的淀粉和蔗糖代谢通路及半胱氨酸和蛋氨酸代谢途径,而中温大曲更多集中在半乳糖、果糖和甘露糖代谢途径等。Yang等[5]利用非靶代谢组学解析了不同类型高温大曲中647种化合物,基于MetaboAnalyst通路富集分析揭示了造成这些化合物显著差异的21条代谢途径是引起大曲代谢表型差异的主要来源,例如苯丙氨酸、酪氨酸和色氨酸的生物合成途径等。Wang等[27]利用BugBase微生物组表型预测和PICRUSt细菌功能潜能预测,结果表明不同类型高温大曲(黄曲、白曲、黑曲)具有相似的功能和表型,其中细菌在高温大曲中代谢旺盛,具有转运和分解氨基酸和碳水化合物的能力。
在堆肥研究中,Kong等[28]利用宏基因组学研究玉米芯堆肥过程中不同理化因素(C/N比、pH、温度和堆肥有机质)与微生物结构之间的关系,发现氨基酸、碳水化合物和脂类代谢是发酵过程中的主要代谢途径,而且这些途径的相对丰度在嗜热阶段显著增加,表明氨基酸、碳水化合物代谢和脂质代谢更加活跃;另外,与萘、氨基苯甲酸盐、阿特拉津和苯甲酸盐降解相关的基因丰度在高温阶段呈上升趋势,说明这些化合物在高温下降解活跃且快速,这可能是由于氨基酸、碳水化合物和脂类在高温下的快速降解,为微生物的代谢提供了足够的能量和碳源。Zhang等[24]将绿色大豆皮和玉米秸秆进行堆肥发酵,也发现碳水化合物代谢和氨基酸代谢是主要的代谢途径,在细菌功能预测结果中碳代谢和氨基酸代谢作为最主要的代谢通路可占到10%以上;其中,碳代谢在纤维素降解起重要作用,氨基酸代谢促进腐殖质合成过程;在真菌代谢预测中属于嗜热真菌属和曲霉属的多个分类单元的相对丰度在高温期占主导地位,具有木质纤维素降解的功能。
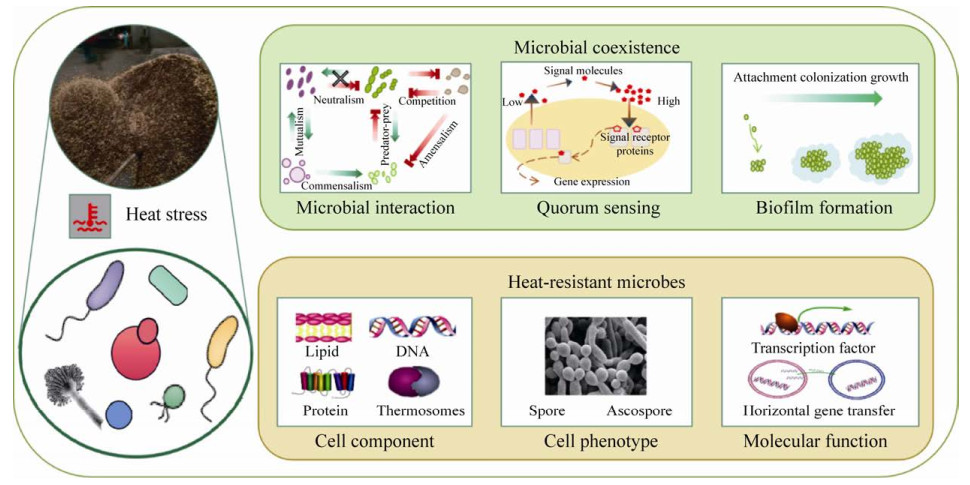
3 发酵菌群高温适应机制生物体经过长期的进化过程发展出各种抗逆机制以适应环境压力。微生物群落由于其生物多样性,可通过调控微生物群落结构、转换相互作用关系及群体形态等抵抗高温,如耐热微生物富集、生物膜形成等;而微生物个体主要通过细胞组分、基因元件的应激变化抵抗高温等(图 2)。
|
| 图 2 固态发酵体系中微生物适应高温的机制解析示意图 Figure 2 The schematic diagram of the mechanism of microbial adaptation to high temperature in solid-state fermentation system. |
|
|
微生物群落的结构和代谢活性对温度变化具有高度的反应性。表 1主要列出了一些发酵样本在高温下的微生物群落组成与代谢功能,表明高温对于大部分固态发酵过程而言,其主要通过富集具有耐热性状的微生物调控代谢途径等,以持续发酵与生长。
| 高温期 High temperature |
发酵样本 Fermented sample |
研究方法 Research methodology |
嗜热阶段微生物组成 Microbial community at the thermophilic phase |
代谢功能 Metabolic function |
参考文献 References |
| T≥70 ℃ | Hyperthermophilic composting | Illumina HiSeq sequencing | Thermaceae, Thermoactinomycetaceae | The formation of continuous high temperature; The enhancement of composting efficiency |
[23] |
| Green soybean hull composting | Illumina MiSeq sequencing, FUNGuild prediction, Tax4Fun prediction | Streptosporangiaceae, Chaetomiaceae | Carbohydrate metabolism; Amino acid metabolism |
[24] | |
| Corncob composting | Metagenomics | Firmicutes (Acinetobacter, Aerococcus, Bacillus, Desemzia, Lysinibacillus, Corynebacterium, Enterococcus) | Carbohydrate metabolism; Lipid metabolism; Amino acid metabolism |
[28] | |
| Manure composting | Illumina MiSeq sequencing, PICRUSt prediction | Firmicutes | Carbohydrate metabolism; Lipid metabolism; Amino acid metabolism |
[29] | |
| 70 ℃ > T≥60 ℃ | Maize straw composting | Illumina MiSeq sequencing, PICRUSt prediction | Sporosarcina, Bacillus | Metabolism, cellular processes and environmental information processing | [20] |
| Jiang-flavor Daqu | Metatranscriptomics | Aspergillus, Penicillium | The starch and sucrose metabolism, and production of potential carbohydrate-active enzymes as saccharifying enzymes | [25] | |
| Jiang-flavor liquor starter | Pyrosequencing | Bacillales, no-rank Actinobacteria, Lactobacillales, Eurotiales, no-rank Eukaryota | The active energy metabolism and carbohydrate digestion metabolism, e.g., starch and sucrose, cysteine and methionine metabolism and terpenoid backbone biosynthesis | [26] | |
| Nong-flavor liquor starter | Pyrosequencing, PICRUSt prediction | Bacillales, Flavobacteriales, Eurotiales, Saccharomycetales, no-rank Eukaryota | The active energy metabolism and carbohydrate digestion metabolism, e.g., galactose, fructose and mannose | [26] | |
| Lignocellulosic waste composting | Metagenomics | Bacteroidetes, Firmicutes | The enhancement of the population of known beneficial microbial groups, the microbial biodiversity, composting rate, and overall final product quality | [30] | |
| Medicinal herbal residues composting | Illumina MiSeq sequencing | Thermomyces, Aspergillus | Lignocellulose degraders | [31] | |
| Food waste composting | Illumina MiSeq sequencing | Firmicutes, Proteobacteria, Bacteroidetes, Acidobacteria | 76% of the overall dioctyl terephthalate (DOTP) degradation | [32] | |
| Maize straw composts | Metaproteomics, pyrosequencing | Thermomyces, Thermobifida, Thermopolyspora |
Lignocellulose degraders | [33] | |
| Aerobic solid-state compost | Metaproteomics, pyrosequencing | Thermobifida, Bacillus, Thermomyces, Aspergillus | The bacterial community for cellulose degradation and the fungal community for hemicellulose degradation | [34] | |
| 60 ℃ > T≥50 ℃ | Medium- temperature Daqu | Illumina MiSeq sequencing | Bacillus, unclassified Streptophyta, Weissella, Thermoactinomyces, Thermoascus, Thermomyces | / | [19] |
| Medium- temperature Daqu | Metagenomics | Thermoactinomyces, Aspergillus, Byssochlamys, Rasamsonia, Talaromyces, Penicillium | The positive effect on the production of volatile compounds | [35] | |
| Cereal starter | Illumina MiSeq sequencing, PCR-DGGE | Bacillus, Aspergillus, Mucor | / | [36] | |
| Pig manure composting | Metabolomics, Illumina MiSeq sequencing | Pseudomonas, Lactobacillus, Bacteroidetes, Flavobacterium, Prevotella | Linoleic acid metabolism | [37] | |
| Daqu | Illumina MiSeq sequencing | Enterobacter, Acinetobacter, Bacillus, Pichia, Wickerhamomyces, Aspergillus, Candida, Clavispora | The improver of enzyme activity, pyrazines and aromatic compounds | [38] | |
| Note: /: The metabolic function of the microbial community during the thermophilic period was not involved in the study. | |||||
微生物通过群落聚集及相互依赖的群落模式对环境扰动表现出良好的抵抗力[39]。在自然系统中,单一物种并不占据一个环境中的所有生态位,而是多个物种共存并发挥互补的作用,形成复杂的生态网络[40-42]。同时,由于环境压力,物种适应性差的微生物的生态位也会被竞争力更强的微生物所占领,进而使整个微生物群落结构变化。固态发酵体系中利用外源或内源微生物通过内在的相互作用等形式可以有效强化高温发酵。Henry等[30]利用嗜热酸性有效微生物提高木质纤维素废弃物堆肥效率,增强了嗜热阶段的微生物多样性和种群数量。Lu等[31]利用多阶段接种功能发酵菌株使中草药残渣堆肥发酵具有最高的嗜热温度(68.2 ℃)和萌发指数(102.68%),加速了纤维素和木质素的降解,也提高了成熟期纤维素酶活性,以及木聚糖酶、锰过氧化物酶和酚类化合物的利用率。
微生物群体感应系统的启动也被用于响应不利环境[43-44]。群体感应可实现多种功能,例如产毒素、产孢、产抗菌素、形成生物膜等。然而,关于嗜热环境下的群体感应机制的信息依然十分有限。Kaur等[45]从106个嗜热真细菌和21个嗜热古菌的蛋白质组数据中发现,AI-1型群体感应系统在这些嗜热菌中不存在,但也发现了一些嗜热菌可能利用LsrB和RbsB操纵子进行AI-2型信号传导,并可能将群体感应用于生物膜形成等目的。生物膜是细菌或真菌在各种不利环境条件(如高酸、高温、高盐等)下发展成被胞外聚合物质包裹的细胞群落的一种常见策略。在固态发酵中,微生物作为生物膜吸附在发酵基质颗粒表面上生长是典型现象,膜下的微观生态系统中微生物和谐共存,协同完成生物化学反应。Almatroudi等[46]研究指出,不同类型的生物膜(浮游生物膜、水合生物膜和干表面生物膜)对温度的敏感性不同,干燥表面生物膜的耐热性更强。此外,Pawluk等[47]也发现无论在体外还是清国酱食物模型中,附着于表面完整生物膜中的蜡样芽孢杆菌孢子的耐热性要强于浮游孢子,表明了生物膜为孢子提供额外保护以抵御热胁迫。尽管关于传统复杂发酵体系中高温条件下群体感应、生物膜等并无直接证据,但其他高温环境下的研究结果加强了人们对传统固态发酵微生物群落耐热性的思考。
关于复杂样本中的群落耐热机制研究较少,更多的研究是在气候变暖大环境下的微生物群落构建方面,通过组学等研究手段研究温度对自然样本中微生物群落的影响。Riah-Anglet等[8]通过热胁迫实验研究不同类型的土壤微生物结构与功能受温度的影响,结果表明热胁迫显著降低了土壤微生物的总量和真菌丰度,并且抑制绝大部分酶活性,然而草地土壤比耕地土壤具有更高的微生物多样性,更能抵抗剧烈的胁迫。García等[41]探讨了不同温度下生物多样性对生物系统功能的影响,结果表明提高生物多样性有助于物种间以更强的功能互补来维持热胁迫下的生态系统生产力。这主要是由于物种个体性状的差异及耐热性状的变化会改变竞争的性质、促进作用和资源分配,从而改变种间互补的程度[41]。
3.2 微生物个体层面的耐热调控传统固态发酵体系涉及众多的微生物种类,包括霉菌、酵母和细菌等。高温环境对不同类型微生物的影响不同。大量的研究已表明微生物生长的上限温度与微生物生物分子的热稳定性紧密相关[48]。例如,细胞膜上含有高饱和度的脂肪酸可以形成强疏水作用,使细胞膜保持稳定性,基因组DNA的高GC含量,热稳定的催化酶,以及特殊的应激保护物质如甘露醇[49]、长链的脂肪酸[50]等。
耐热孢子是微生物对抗高温等不利环境下的休眠体。芽孢杆菌、丝状真菌是固态发酵中形成耐热孢子的主要来源。细菌性芽孢一般在停止正常的分裂生长后经过浓缩脱水分后形成,芽孢外壁的主要成分是蛋白质、肽聚糖等,并且含有较高的2, 6-吡啶二羧酸钙,芽孢内几乎不进行代谢,酶活性也很低,并且在芽孢核心区域一般pH值都较低[51]。部分芽孢达到100 ℃以上才能致死。耐热子囊孢子是丝状真菌耐热性的基础[52]。Thermoascus产生的耐热孢子可以在90 ℃存活[53]。
微生物通过调控细胞体内的微环境来保护自身免受高温的冲击。微生物的耐热性属于复杂表型,会涉及多个基因的协同作用。Wu等[54]利用微阵列技术发现,热休克蛋白的响应性增加可用于维持Bacillus licheniformis在高温下的生存。此外,热胁迫也是影响酵母固态发酵产乙醇的重要限制因素。很多研究证明了通过过表达内源性或外源性胁迫相关转录因子基因可有效提高宿主的胁迫耐受性,促进高温下的细胞生长,以提高目标物产量[55-56]。
微生物也可以通过基因组的变异来适应高温环境。有些研究发现了微生物中耐热遗传元件的水平转移现象。例如,耐热性操纵子spoVA 2mob和耐热基因岛LHR分别赋予细菌内生孢子和肠杆菌科耐热性[57-59]。由于LHR和spoVA 2mob操纵子都是可移动的遗传元件,可在细菌间进行水平转移。Wang等[60]发现随着大曲固态发酵的进行,菌群中的LHR和spoVA 2mob操纵子的丰度相较于细菌16S rRNA基因的丰度分别增加了3倍和5倍,表明耐热芽孢杆菌和肠杆菌被自发选择以适应高温环境。此外,Caspeta等[61]通过适应性进化策略选择在高于40 ℃条件下生长良好且高产乙醇的酵母菌株,并利用全基因组测序、全基因组基因表达和代谢通量分析,揭示了由C-5甾醇去饱和酶基因突变引起的甾醇组成变化(从麦角甾醇到粪甾醇),以及参与甾醇生物合成的基因表达增加从而改变了酵母的耐热性。Wang等[62]利用聚合全基因组测序的数量性状位点定位分析揭示了酵母基因组上的变异,发现致病基因杂合度的降低有助于提高高温发酵能力,而且3个优越亲本等位基因(VPS34、VID24和DAP1)相对于其劣势亲本等位基因而言更有利于高温发酵,其生理影响主要通过增加海藻糖积累或降低膜流动性而提高耐热性。
以上内容表明微生物群落结构组成、多样性、微生物间相互作用以及微生物的生理性状、遗传特征等均可能是潜在的耐热机制。然而,群体耐热机制可能涉及更深层次的机制,不仅是目前所认知的简单的相互作用关系、群体感应等可能性的机理,还包括生态位分化、微生物高阶互作等行为。同样地,微生物群落对高温的适应体现在群落、细胞、基因和代谢等方面,是一个复杂的多层次调控过程。
4 总结与展望固态发酵体系中生物热的产生影响着微生物群落演替及其代谢。在生物热驱动的发酵体系中往往会积累大量的耐热、嗜热微生物。这种高温适应性除了来源于微生物的细胞结构,如利用抗高温元件的生物大分子(蛋白质、磷脂分子),还包括细胞内的代谢调控等。此外,混菌体系下的微生物相互作用也起到了潜在的耐热作用。了解高温下微生物代谢调控和发酵的规律,并运用工程概念来分析和调控发酵过程,有望实现微生物高温发酵控制,以达到更高产率的生产与降解的目标。目前仍有许多基础工作有待完善:
(1) 解析高温环境下微生物群落代谢机制
固态发酵微生物群落具有高度的复杂性,了解微生物群体代谢机制是一个巨大的挑战。解析微生物群落代谢行为需要以整体发酵任务为研究基础,通过系统生物学、多组学技术和生物信息学技术来构建发酵菌群表型与基因型的交互关系,从而明确关键代谢节点的核心微生物,这将是多维度解析微生物群落功能的有效途径[16, 18, 63]。此外,通过可培养技术了解更多有效物种的代谢特征,以验证分析微生物群落的生态过程[17, 30]。高温胁迫环境下更应该关注微生物群落潜在的相互作用所引起的代谢交互[8, 41]。
(2) 功能性耐热、嗜热微生物的挖掘及其固态发酵中的应用
尽管高通量测序已成为目前表征微生物群落多样性最主要的研究工具,然而对于实际应用而言,嗜热菌和耐热真菌的分离培养依然是必要的,功能性菌株的挖掘是微生物发酵行业持续发展的前提[52, 64]。大部分自然界中的微生物是不可培养或难以培养的,因而可培养组学和原位培养技术(膜扩散培养、微流体培养、胶囊包埋等)的开发也为获取未培养微生物提供了有效的挖掘工具[65]。
(3) 基于环境协调控制微生物代谢,有效提高目标产物的产量及转化效率
调控微生物群落的功能以明确发酵微生物的代谢特征为前提,从而确定微生物的分工模式。另外,在不破坏封闭发酵体系的前提下有效收集发酵过程中参数,从而评估发酵过程和生产效果,完善对发酵机制的认识,进而更合理地指导生产方案,以提高发酵目标产物的优质率[12, 66]。同时,构建数学模型、预测模型及应用是未来发酵产品定制化生产的重要发展方向,过程控制是稳定发酵的基本保证。
| [1] |
Xu Y. Modern Liquor Brewing Microbiology[M]. Beijing: Science Press, 2019. (in Chinese) 徐岩. 现代白酒酿造微生物学[M]. 北京: 科学出版社, 2019. |
| [2] |
Ren C, Du H, Xu Y. Advances in microbiome study of traditional Chinese fermented foods[J]. Acta Microbiologica Sinica, 2017, 57(6): 885-898. (in Chinese) 任聪, 杜海, 徐岩. 中国传统发酵食品微生物组研究进展[J]. 微生物学报, 2017, 57(6): 885-898. |
| [3] |
Wu Q, Zhu Y, Fang C, Wijffels RH, Xu Y. Can we control microbiota in spontaneous food fermentation: Chinese liquor as a case example[J]. Trends in Food Science & Technology, 2021, 110: 321-331. |
| [4] |
Wang XS, Du H, Zhang Y, Xu Y. Environmental microbiota drives microbial succession and metabolic profiles during Chinese liquor fermentation[J]. Applied and Environmental Microbiology, 2018, 84(4): e02369-e02317. |
| [5] |
Yang L, Fan WL, Xu Y. GC×GC-TOF/MS and UPLC-Q-TOF/MS based untargeted metabolomics coupled with physicochemical properties to reveal the characteristics of different type daqus for making soy sauce aroma and flavor type baijiu[J]. LWT, 2021, 146: 111416. DOI:10.1016/j.lwt.2021.111416 |
| [6] |
Zhang HX, Wang L, Wang HY, Yang F, Chen LQ, Hao F, Lyu XB, Du H, Xu Y. Effects of initial temperature on microbial community succession rate and volatile flavors during Baijiu fermentation process[J]. Food Research International, 2021, 141: 109887. DOI:10.1016/j.foodres.2020.109887 |
| [7] |
Chen HZ. Modern Solid State Fermentation[M]. Springer, 2013
|
| [8] |
Riah-Anglet W, Trinsoutrot-Gattin I, Martin-Laurent F, Laroche-Ajzenberg E, Norini MP, Latour X, Laval K. Soil microbial community structure and function relationships: a heat stress experiment[J]. Applied Soil Ecology, 2015, 86: 121-130. DOI:10.1016/j.apsoil.2014.10.001 |
| [9] |
Wipf HML, Bùi TN, Coleman-Derr D. Distinguishing between the impacts of heat and drought stress on the root microbiome of Sorghum bicolor[J]. Phytobiomes Journal, 2021, 5(2): 166-176. DOI:10.1094/PBIOMES-07-20-0052-R |
| [10] |
Moeller AH, Ivey K, Cornwall MB, Herr K, Rede J, Taylor EN, Gunderson AR. The lizard gut microbiome changes with temperature and is associated with heat tolerance[J]. Applied and Environmental Microbiology, 2020, 86(17): e01181-e01120. |
| [11] |
Xie W, Zhou LJ, Xu J, Guo QL, Jiang FL, Liu Y. Advances in biothermochemistry and thermokinetics[J]. Acta Physico-Chimica Sinica, 2020, 36(6): 19-28. (in Chinese) 谢文, 周莲娇, 徐娟, 郭清莲, 蒋风雷, 刘义. 生物热化学和热动力学研究进展[J]. 物理化学学报, 2020, 36(6): 19-28. |
| [12] |
Jin GY, Uhl P, Zhu Y, Wijffels RH, Xu Y, Rinzema A. Modeling of industrial-scale anaerobic solid-state fermentation for Chinese liquor production[J]. Chemical Engineering Journal, 2020, 394: 124942. DOI:10.1016/j.cej.2020.124942 |
| [13] |
Zhang YZ, Wang L, Chen HZ. Correlations of medium physical properties and process performance in solid-state fermentation[J]. Chemical Engineering Science, 2017, 165: 65-73. DOI:10.1016/j.ces.2017.02.039 |
| [14] |
Wang SL, Xiong W, Wang YQ, Nie Y, Wu Q, Xu Y, Geisen S. Temperature-induced annual variation in microbial community changes and resulting metabolome shifts in a controlled fermentation system[J]. mSystems, 2020, 5(4): e00555-e00520. |
| [15] |
Wang BW, Wu Q, Xu Y, Sun BG. Multiple sugars promote microbial interactions in Chinese baijiu fermentation[J]. LWT, 2021, 138: 110631. DOI:10.1016/j.lwt.2020.110631 |
| [16] |
Blasche S, Kim Y, Mars RAT, Machado D, Maansson M, Kafkia E, Milanese A, Zeller G, Teusink B, Nielsen J, et al. Metabolic cooperation and spatiotemporal niche partitioning in a kefir microbial community[J]. Nature Microbiology, 2021, 6(2): 196-208. DOI:10.1038/s41564-020-00816-5 |
| [17] |
Zhang HX, Du H, Xu Y. Volatile organic compound-mediated antifungal activity of Pichia spp. and its effect on the metabolic profiles of fermentation communities[J]. Applied and Environmental Microbiology, 2021, 87(9). |
| [18] |
Yang L, Fan WL, Xu Y. Metaproteomics insights into traditional fermented foods and beverages[J]. Comprehensive Reviews in Food Science and Food Safety, 2020, 19(5): 2506-2529. DOI:10.1111/1541-4337.12601 |
| [19] |
Xiao C, Lu ZM, Zhang XJ, Wang ST, Ao L, Shen CH, Shi JS, Xu ZH. Bio-heat is a key environmental driver shaping the microbial community of mediumtemperature Daqu[J]. Applied and Environmental Microbiology, 2017, 83(23): e01550-e01517. |
| [20] |
Wei HW, Wang LH, Hassan M, Xie B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process[J]. Bioresource Technology, 2018, 256: 333-341. DOI:10.1016/j.biortech.2018.02.050 |
| [21] |
Wang BW, Wu Q, Xu Y, Sun BG. Recent advances and perspectives in study of microbiome in Chinese Jiuqu starter[J]. Microbiology China, 2021, 48(5): 1737-1746. (in Chinese) 王柏文, 吴群, 徐岩, 孙宝国. 中国白酒酒曲微生物组研究进展及趋势[J]. 微生物学通报, 2021, 48(5): 1737-1746. |
| [22] |
Fang C, Du H, Xu Y. Diversity of Daqu filamentous fungi and their potentials for synthesizing bioactive compounds[J]. Food and Fermentation Industries, 2019, 45(15): 1-8. (in Chinese) 方程, 杜海, 徐岩. 大曲丝状真菌的物种多样性及其次级代谢产物的合成潜力[J]. 食品与发酵工业, 2019, 45(15): 1-8. |
| [23] |
Yu Z, Tang J, Liao HP, Liu XM, Zhou PX, Chen Z, Rensing C, Zhou SG. The distinctive microbial community improves composting efficiency in a full-scale hyperthermophilic composting plant[J]. Bioresource Technology, 2018, 265: 146-154. DOI:10.1016/j.biortech.2018.06.011 |
| [24] |
Zhang C, Gao Z, Shi WC, Li LC, Tian RM, Huang J, Lin RS, Wang B, Zhou B. Material conversion, microbial community composition and metabolic functional succession during green soybean hull composting[J]. Bioresource Technology, 2020, 316: 123823. DOI:10.1016/j.biortech.2020.123823 |
| [25] |
Yi ZL, Jin YL, Xiao Y, Chen LC, Tan L, Du AP, He KZ, Liu DY, Luo HB, Fang Y, et al. Unraveling the contribution of high temperature stage to Jiang-flavor Daqu, a liquor starter for production of Chinese Jiang-flavor baijiu, with special reference to metatranscriptomics[J]. Frontiers in Microbiology, 2019, 10: 472. DOI:10.3389/fmicb.2019.00472 |
| [26] |
Huang Y, Yi Z, Jin Y, Zhao Y, He K, Liu D, Zhao D, He H, Luo H, Zhang W, et al. New microbial resource: microbial diversity, function and dynamics in Chinese liquor starter[J]. Scientific Reports, 2017, 7: 14577. DOI:10.1038/s41598-017-14968-8 |
| [27] |
Wang YR, Cai WC, Wang WP, Shu N, Zhang ZD, Hou QC, Shan CH, Guo Z. Analysis of microbial diversity and functional differences in different types of high-temperature Daqu[J]. Food Science & Nutrition, 2021, 9(2): 1003-1016. |
| [28] |
Kong WL, Sun B, Zhang JY, Zhang YT, Gu LK, Bao LJ, Liu SX. Metagenomic analysis revealed the succession of microbiota and metabolic function in corncob composting for preparation of cultivation medium for Pleurotus ostreatus[J]. Bioresource Technology, 2020, 306: 123156. DOI:10.1016/j.biortech.2020.123156 |
| [29] |
Bao YY, Feng YZ, Qiu CW, Zhang JW, Wang YM, Lin XG. Organic matter- and temperature-driven deterministic assembly processes govern bacterial community composition and functionality during manure composting[J]. Waste Management, 2021, 131: 31-40. DOI:10.1016/j.wasman.2021.05.033 |
| [30] |
Henry AB, Maung CEH, Kim KY. Metagenomic analysis reveals enhanced biodiversity and composting efficiency of lignocellulosic waste by thermoacidophilic effective microorganism (tEM)[J]. Journal of Environmental Management, 2020, 276: 111252. DOI:10.1016/j.jenvman.2020.111252 |
| [31] |
Lu XL, Wu H, Song SL, Bai HY, Tang MJ, Xu FJ, Ma Y, Dai CC, Jia Y. Effects of multi-phase inoculation on the fungal community related with the improvement of medicinal herbal residues composting[J]. Environmental Science and Pollution Research International, 2021, 28(22): 27998-28013. DOI:10.1007/s11356-021-12569-7 |
| [32] |
Tran HT, Lin C, Bui XT, Itayama T, Dang BT, Cheruiyot NK, Hoang HG, Vu CT. Bacterial community progression during food waste composting containing high dioctyl terephthalate (DOTP) concentration[J]. Chemosphere, 2021, 265: 129064. DOI:10.1016/j.chemosphere.2020.129064 |
| [33] |
Zhang LL, Ma HX, Zhang HQ, Xun LY, Chen GJ, Wang LS. Thermomyces lanuginosus is the dominant fungus in maize straw composts[J]. Bioresource Technology, 2015, 197: 266-275. DOI:10.1016/j.biortech.2015.08.089 |
| [34] |
Zhang LL, Zhang HQ, Wang ZH, Chen GJ, Wang LS. Dynamic changes of the dominant functioning microbial community in the compost of a 90-m3 aerobic solid state fermentor revealed by integrated meta-omics[J]. Bioresource Technology, 2016, 203: 1-10. DOI:10.1016/j.biortech.2015.12.040 |
| [35] |
Yang Y, Wang ST, Lu ZM, Zhang XJ, Chai LJ, Shen CH, Shi JS, Xu ZH. Metagenomics unveils microbial roles involved in metabolic network of flavor development in medium-temperature Daqu starter[J]. Food Research International, 2021, 140: 110037. DOI:10.1016/j.foodres.2020.110037 |
| [36] |
Li P, Liang HB, Lin WT, Feng F, Luo LX. Microbiota dynamics associated with environmental conditions and potential roles of cellulolytic communities in traditional Chinese cereal starter solid-state fermentation[J]. Applied and Environmental Microbiology, 2015, 81(15): 5144-5156. DOI:10.1128/AEM.01325-15 |
| [37] |
Liu LX, Wang TZ, Li SS, Hao RR, Li QH. Combined analysis of microbial community and microbial metabolites based on untargeted metabolomics during pig manure composting[J]. Biodegradation, 2021, 32(2): 217-228. DOI:10.1007/s10532-021-09935-0 |
| [38] |
Wang P, Wu Q, Jiang XJ, Wang ZQ, Tang JL, Xu Y. Bacillus licheniformis affects the microbial community and metabolic profile in the spontaneous fermentation of Daqu starter for Chinese liquor making[J]. International Journal of Food Microbiology, 2017, 250: 59-67. DOI:10.1016/j.ijfoodmicro.2017.03.010 |
| [39] |
Wang M, Liu X, Nie Y, Wu XL. Selfishness driving reductive evolution shapes interdependent patterns in spatially structured microbial communities[J]. The ISME Journal, 2021, 15(5): 1387-1401. DOI:10.1038/s41396-020-00858-x |
| [40] |
Lopatkin AJ, Collins JJ. Predictive biology: modelling, understanding and harnessing microbial complexity[J]. Nature Reviews Microbiology, 2020, 18(9): 507-520. DOI:10.1038/s41579-020-0372-5 |
| [41] |
García FC, Bestion E, Warfield R, Yvon-Durocher G. Changes in temperature alter the relationship between biodiversity and ecosystem functioning[J]. PNAS, 2018, 115(43): 10989-10994. DOI:10.1073/pnas.1805518115 |
| [42] |
Malik AA, Martiny JBH, Brodie EL, Martiny AC, Treseder KK, Allison SD. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change[J]. The ISME Journal, 2020, 14(1): 1-9. DOI:10.1038/s41396-019-0510-0 |
| [43] |
Johansen P, Jespersen L. Impact of quorum sensing on the quality of fermented foods[J]. Current Opinion in Food Science, 2017, 13: 16-25. DOI:10.1016/j.cofs.2017.01.001 |
| [44] |
Monnet V, Gardan R. Quorum-sensing regulators in Gram-positive bacteria: 'cherchez le peptide'[J]. Molecular Microbiology, 2015, 97(2): 181-184. DOI:10.1111/mmi.13060 |
| [45] |
Kaur A, Capalash N, Sharma P. Quorum sensing in thermophiles: prevalence of autoinducer-2 system[J]. BMC Microbiology, 2018, 18(1): 62. DOI:10.1186/s12866-018-1204-x |
| [46] |
Almatroudi A, Tahir S, Hu H, Chowdhury D, Gosbell IB, Jensen SO, Whiteley GS, Deva AK, Glasbey T, Vickery K. Staphylococcus aureus dry-surface biofilms are more resistant to heat treatment than traditional hydrated biofilms[J]. The Journal of Hospital Infection, 2018, 98(2): 161-167. DOI:10.1016/j.jhin.2017.09.007 |
| [47] |
Pawluk AM, Kim D, Jin YH, Jeong KC, Mah JH. Biofilm-associated heat resistance of Bacillus cereus spores in vitro and in a food model, Cheonggukjang jjigae[J]. International Journal of Food Microbiology, 2022, 363: 109505. DOI:10.1016/j.ijfoodmicro.2021.109505 |
| [48] |
Zeng J, Guo JJ, Qiu XZ, Wang XZ, Yuan L. Advances on hyperthermophiles and mechanism of their thermal adaptation[J]. Biotechnology Bulletin, 2015, 31(9): 30-37. (in Chinese) 曾静, 郭建军, 邱小忠, 王贤卓, 袁林. 极端嗜热微生物及其高温适应机制的研究进展[J]. 生物技术通报, 2015, 31(9): 30-37. |
| [49] |
Frąc M, Jezierska-Tys S, Yaguchi T. Occurrence, detection, and molecular and metabolic characterization of heat-resistant fungi in soils and plants and their risk to human health[J]. Advances in Agronomy, 2015, 132: 161-204. |
| [50] |
Li PS, Tan XW, Fu XF, Dang Y, Li SZ. Metabolomic analysis reveals Kluyveromyces marxianus's stress responses during high-temperature ethanol fermentation[J]. Process Biochemistry, 2021, 102: 386-392. DOI:10.1016/j.procbio.2021.01.024 |
| [51] |
Wang WY, Liu RL, Shen Y, Lian B. The potential correlation between bacterial sporulation and the characteristic flavor of Chinese Maotai liquor[J]. Frontiers in Microbiology, 2018, 9: 1435. DOI:10.3389/fmicb.2018.01435 |
| [52] |
Witfeld F, Begerow D, Guerreiro MA. Improved strategies to efficiently isolate thermophilic, thermotolerant, and heat-resistant fungi from compost and soil[J]. Mycological Progress, 2021, 20(3): 325-339. DOI:10.1007/s11557-021-01674-z |
| [53] |
Scaramuzza N, Berni E. Heat-resistance of Hamigera avellanea and Thermoascus crustaceus isolated from pasteurized acid products[J]. International Journal of Food Microbiology, 2014, 168/169: 63-68. DOI:10.1016/j.ijfoodmicro.2013.10.007 |
| [54] |
Wu Q, Xu Y. Transcriptome profiling of heat-resistant strain Bacillus licheniformis CGMCC3962 producing Maotai flavor[J]. Journal of Agricultural and Food Chemistry, 2012, 60(8): 2033-2038. DOI:10.1021/jf204270h |
| [55] |
Li PS, Fu XF, Zhang L, Zhang ZY, Li JH, Li SZ. The transcription factors Hsf1 and Msn2 of thermotolerant Kluyveromyces marxianus promote cell growth and ethanol fermentation of Saccharomyces cerevisiae at high temperatures[J]. Biotechnology for Biofuels, 2017, 10: 289. DOI:10.1186/s13068-017-0984-9 |
| [56] |
Ge XY, Xu Y, Chen X. Improve carbon metabolic flux in Saccharomyces cerevisiae at high temperature by overexpressed TSL1 gene[J]. Journal of Industrial Microbiology and Biotechnology, 2013, 40(3/4): 345-352. |
| [57] |
Mercer RG, Zheng JS, Garcia-Hernandez R, Ruan LF, Gänzle MG, McMullen LM. Genetic determinants of heat resistance in Escherichia coli[J]. Frontiers in Microbiology, 2015, 6: 932. |
| [58] |
Berendsen EM, Boekhorst J, Kuipers OP, Wells-Bennik MHJ. A mobile genetic element profoundly increases heat resistance of bacterial spores[J]. The ISME Journal, 2016, 10(11): 2633-2642. DOI:10.1038/ismej.2016.59 |
| [59] |
Krawczyk AO, De Jong A, Omony J, Holsappel S, Wells-Bennik MHJ, Kuipers OP, Eijlander RT. Spore heat activation requirements and germination responses correlate with sequences of germinant receptors and with the presence of a specific spoVA2mob operon in foodborne strains of Bacillus subtilis[J]. Applied and Environmental Microbiology, 2017, 83(7): e03122-e03116. |
| [60] |
Wang ZY, Li P, Luo LX, Simpson DJ, Gänzle MG. Daqu fermentation selects for heat-resistant Enterobacteriaceae and bacilli[J]. Applied and Environmental Microbiology, 2018, 84(21): e01483-e01418. |
| [61] |
Caspeta L, Chen Y, Ghiaci P, Feizi A, Buskov S, Hallström BM, Petranovic D, Nielsen J. Altered sterol composition renders yeast thermotolerant[J]. Science, 2014, 346(6205): 75-78. DOI:10.1126/science.1258137 |
| [62] |
Wang Z, Qi Q, Lin YP, Guo YF, Liu YF, Wang QH. Correction to: QTL analysis reveals genomic variants linked to high-temperature fermentation performance in the industrial yeast[J]. Biotechnology for Biofuels, 2019, 12: 83. DOI:10.1186/s13068-019-1425-8 |
| [63] |
Wang SL, Wu Q, Nie Y, Wu JF, Xu Y. Construction of synthetic microbiota for reproducible flavor compound metabolism in Chinese light-aroma-type liquor produced by solid-state fermentation[J]. Applied and Environmental Microbiology, 2019, 85(10): e03090-e03018. |
| [64] |
Atalah J, Cáceres-Moreno P, Espina G, Blamey JM. Thermophiles and the applications of their enzymes as new biocatalysts[J]. Bioresource Technology, 2019, 280: 478-488. DOI:10.1016/j.biortech.2019.02.008 |
| [65] |
Lewis WH, Tahon G, Geesink P, Sousa DZ, Ettema TJG. Innovations to culturing the uncultured microbial majority[J]. Nature Reviews Microbiology, 2021, 19(4): 225-240. DOI:10.1038/s41579-020-00458-8 |
| [66] |
Du RB, Ren C, Wu Q, Xu Y. The ecological fermentation technology: principle and its applications[J]. Food and Fermentation Industries, 2021, 47(1): 266-275. (in Chinese) 杜如冰, 任聪, 吴群, 徐岩. 生态发酵技术原理与应用[J]. 食品与发酵工业, 2021, 47(1): 266-275. |
 2022, Vol. 49
2022, Vol. 49