扩展功能
文章信息
- 张念琦, 何展, 程昌勇, 宋厚辉
- ZHANG Nianqi, HE Zhan, CHENG Changyong, SONG Houhui
- 病原细菌调控丝裂原激活蛋白激酶信号介导先天免疫的机制研究进展
- Innate immune mechanism mediated by MAPK signaling pathway during bacterial infection
- 微生物学通报, 2022, 49(12): 5298-5310
- Microbiology China, 2022, 49(12): 5298-5310
- DOI: 10.13344/j.microbiol.china.220920
-
文章历史
- 收稿日期: 2022-09-22
- 接受日期: 2022-11-13
- 网络首发日期: 2022-11-28
宿主产生的先天免疫反应依靠数量有限的模式识别受体(pattern recognition receptor,PRRs)识别微生物固定成分的病原体相关分子模式(pathogen-associated molecular patterns,PAMPs),是宿主被感染后的首道抵抗防线[1-2]。PRRs接受刺激后在细胞内诱导多种靶向病原蛋白(如抗菌肽)或其他促炎因子(如细胞因子、趋化因子)的转录表达。巨噬细胞和树突状细胞(dendritic cells,DCs)是炎症反应的关键介质,这两类细胞能够表达多种类型的PRRs,包括Toll样受体(Toll-like receptors,TLRs)、RIG-I样受体(RLG-I-like receptors,RLRs)、NOD样受体(NOD-like receptors,NLRs)和C型凝集素受体(C-type lectin receptors,CLRs)[3-4]。这些PRRs接受不同刺激但都能够激活丝裂原激活蛋白激酶(mitogen-activated protein kinase,MAPK)和核因子κB (nuclear factor-κB,NF-κB)通路[5-6];而MAPK途径的激酶是炎症反应的关键参与者,对于保护宿主平衡起到重要作用[7-8]。这也使得细菌和病毒会选择MAPK信号通路为靶点来确保在宿主固有和适应性免疫系统下存活。通过研究免疫反应启动、调节过程中宿主-微生物相互作用机制有助于揭示微生物病原体引起感染性疾病的方法,而且为细胞调控信号传导机制提供了有价值的工具。
MAPK途径的功能和结构从单细胞生物(如酵母)到复杂生物(包括哺乳动物)进化过程中一直是保守的,主要是将上游信号传递至其下游效应子,调节许多细胞关键的生理过程,如细胞增殖、先天免疫反应、细胞迁移、凋亡和自噬等,其中,TLRs在介导MAPK信号激活中发挥重要作用[9-10]。
鉴于MAPK信号通路在调节免疫反应中的重要性,许多细菌病原体已经发展出直接或间接调节MAPK激活或抑制机制[11-13]。这些病原体通常使用效应蛋白来操纵MAPK途径,并允许细菌在宿主内建立感染。本实验室前期研究表明,单增李斯特菌感染会触发ERK1/2和p38信号传导的激活,并且这种细胞反应是单增李斯特菌感染所必需的;单增李斯特菌感染上皮细胞可以通过李斯特菌溶血素O (listeriolysin O,LLO)的作用激活MAPK激酶的磷酸化;同时,LLO还有助于李斯特菌感染滋养层细胞,通过MAPK家族蛋白去磷酸化来抑制MAPK信号通路激活[14]。与其他胆固醇结合细胞溶素(cholesterol dependent cytolysin,CDC)家族成员不同,LLO显示出限制其细胞毒性的特征[15-16]。然而,LLO的孔形成活性与操纵MAPK通路的能力之间的相关性尚不清楚,LLO在MAPK通路信号传导中的详细机制需要进一步研究。
1 TLRs概述哺乳动物的抗原呈递细胞如树突状细胞和巨噬细胞表达的TLRs是关键的模式识别受体(pattern recognition receptor,PRR),在诱导先天免疫应答及适应性免疫应答中起着核心作用[17-18]。该识别机制依赖不同的TLRs识别细菌PAMPs。例如,TLR2、TLR4和TLR5是位于细胞表面的TLR,分别识别细菌脂蛋白、脂多糖和鞭毛蛋白,而胞内TLR如TLR8识别病毒和细菌RNA,并优先被富含AU的ssRNA激活(图 1)[17, 19-20]。
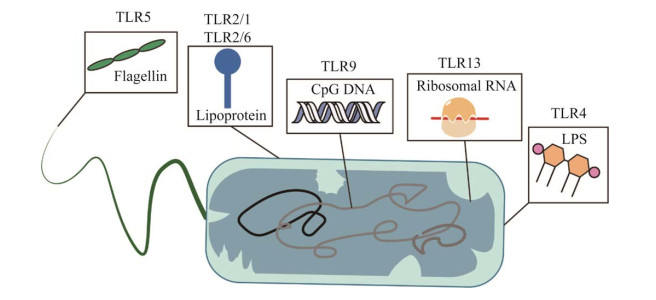
|
| 图 1 Toll样受体(TLRs)可特异性识别病原体相关分子模式(PAMPS) Figure 1 TLRs specifically recognize PAMPs. |
|
|
先天免疫细胞中MAPK激活过程几乎都是以TLRs充当激动剂为前提进行。TLRs主要通过2种衔接蛋白发出信号,即β干扰素TIR结构域接头蛋白(TIR domain-containing adaptor protein inducing interferon β,TRIF)和髓样分化初级反应蛋白88 (myeloid differentiation primary-response protein 88,MyD88)[21-22]。两种衔接蛋白存在特异性和偏好性差异,MyD88主要激活NF-κB和MAPK,倾向于产生促炎症细胞因子如IL-6和IL-12来诱导促炎反应,而TRIF主要激活干扰素调节因子(interferon regulator factor,IRF)家族成员,并倾向于诱导干扰素来刺激抗病毒反应;在配体与TLRs结合后,白介素-1受体相关激酶4 (interleukin-1 receptor-associated kinase 4,IRAK4)才被募集到MyD88;随后,IRAK4与IRAK1、IRAK2、E3泛素连接酶肿瘤坏死因子受体相关因子6 [tumor necrosis factor (TNF) receptor associated factor 6,TRAF6]和E2泛素结合酶E2N (ubiquitin-conjugating enzyme E2N,UBE2N)形成复合体[23-24]。然而,TRAF6和UBE2N催化TRAF6和IRAK1上K63多泛素链的形成,也进一步激活MAPK和NF-κB通路[25]。除了TLR,其他3个PRR家族包括CLRs、RLRs和NLRs,虽然受体近端激活机制及其定位存在差异,但同样能够激活MAPK和NF-κB信号通路[26-27]。
2 MAPK途径概述MAPK信号级联主要由保守的三级结构组成,分别是MAP3K、MAP2K和MAPK (图 2)。其中,MAP2K通过Thr-X-Tyr激活基序的双磷酸化激活MAPK (其中X代表任意氨基酸)[28]。哺乳动物细胞表达14种MAPKs,根据序列同源性,可将这些MAPKs细分成若干组[29-30]。
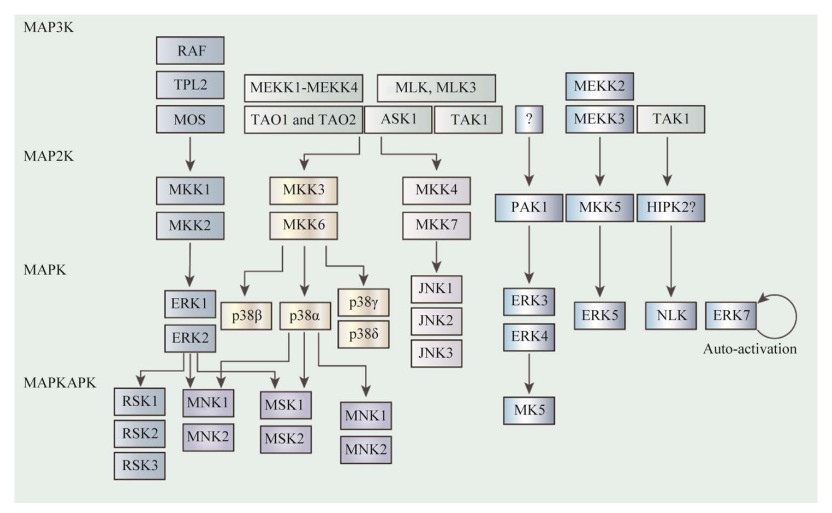
|
| 图 2 MAPK信号通路示意图 Figure 2 MAPK signaling pathways. MAPK途径由一系列激酶组成,至少包含3种激酶MAP3K、MAP2K和MAPK;MAP2K能通过Thr-X-Tyr活化基序的双重磷酸化激活MAPK (其中X代表任意氨基酸);哺乳动物细胞能表达14种MAPK,这些MAPK依据序列同源性分成不同组别 MAPK pathways consist of at least three kinases: MAP3K, MAP2K and MAPK. MAPK kinase (MAP2K) activates the MAPK by dual phosphorylation of the Thr-X-Tyr activation motif (where X represents any amino acid). Mammalian cells express 14 MAPKs, and these can be subdivided into groups based on sequence homology. |
|
|
经典的MAPKs是细胞外信号调节激酶1/2 (extracellular signal-regulated kinase 1/2,ERK1/2),由MKK1或MKK2激活[31]。在生长因子和抗原受体刺激后,快速加速的纤维肉瘤(rapidly accelerated fibrosarcoma,RAF)蛋白在这一级联反应中起MAP3K作用,但在先天免疫应答中,肿瘤进展位点2 (tumour progression locus 2,TPL2)则作为MAP3K起主导作用,同时能够被TLRs、肿瘤坏死因子受体1 (tumor necrosis factor receptor 1,TNFR1)和白细胞介素1受体(interleukin-1 receptor,IL-1R)所用[30]。
p38 MAPK家族包括4种亚型(p38α、p38β、p38γ和p38δ),由MAP2Ks的MKK3和MKK6激活[30]。Jun N末端激酶(jun N-terminal kinase,JNK)家族有3种亚型(JNK1、JNK2和JNK3),由MKK4和MKK7激活[32]。许多MAP3Ks,包括MAP3K/ERK激酶激酶(MAPK/ERK kinase kinases,MEKKs)、TAO1、TAO2、凋亡信号调节激酶1 (apoptosis signal-regulating kinase 1,ASK1)等都能够激活p38和JNK级联,而所需的特定MAP3K取决于刺激和细胞类型[29]。
ERK5级联包括MEKK2或MEKK3-MKK5- ERK5这三部分。p21活化激酶1 (p21-activated kinase 1,PAK1)在ERK3和ERK4上游起作用,而ERK7在寡聚后发生自我激活[33-34]。NEMO样激酶(NEMO-like kinase,NLK)的激活机制尚不清楚,但同源结构域相互作用蛋白激酶2 (homeodomain-interacting protein kinase 2,HIPK2)被认为是TAK1下游潜在的激活因子,并能与之相互作用[33]。一些激酶在MAPKs下游被激活,包括有丝分裂原和应激激活激酶(ribosomal protein S6 kinases,MKs),MAPK信号整合激酶1 (MAPK signal- integrating kinase 1,MNK1)和核糖体蛋白S6激酶(ribosomal protein S6 kinases,RSKs)等[35]。
3 MAPK级联激活MAPK信号通路级联是由3个激酶组成的核心层,其中上游激酶负责磷酸化和下游激酶的激活(图 3)。典型的MAPK激活是通过双特异性MAPK激酶(MAP2K)对激活环中的Thr-X-Tyr基序的双磷酸化介导的[29, 31]。体外研究和过表达研究表明,MKK4和MKK7优先磷酸化Jun N末端激酶(JNKs) Thr-X-Tyr基序中的酪氨酸和苏氨酸残基[29]。然而,内源性MKK4和MKK7对JNK激活表现更为复杂。使用MKK4缺陷和MKK7缺陷小鼠胚胎成纤维细胞(mouse embryo fibroblasts,MEF)分析不同的支架蛋白通过募集不同的下游分子在各种信号通路中发挥不同的作用;支架蛋白Axin主要依赖于MKK7来激活JNK,而支架蛋白Dvl几乎同样依赖于MKK4和MKK7来激活JNK,相比之下,LMP-1诱导的JNK激活主要依赖于MKK4[36]。
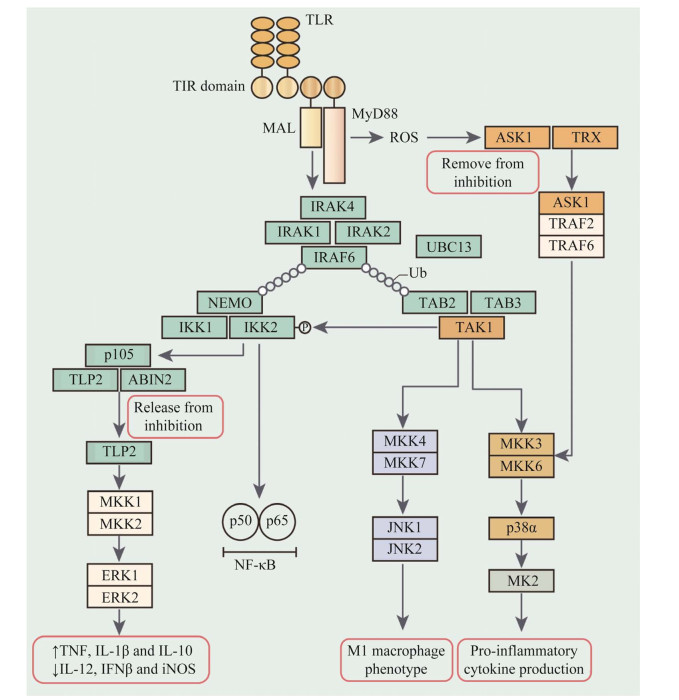
|
| 图 3 MAPK途径的激活与MyD88和TRIF有关 Figure 3 Activation of MAPK is associated with TRIF and MyD88. 在TLR或IL-1R连接之后,MyD88结合到受体的Toll和TIR结构域。对于TLR2和TLR4,MyD88的结合需要衔接蛋白样蛋白(MAL也称为TIRAP),MyD88和丝氨酸/苏氨酸激酶IRAK4通过死亡结构域发生相互作用;IRAK4与IRAK1、IRAK2、E3泛素连接酶TRAF6结合形成多聚复合物进而发挥活性作用;UBC13与TRAF6或其他E3连接酶结合可催化K63连接的多聚泛素链上的TRAF6与IRAK1的结合;TAK1结合蛋白2 (TAB2)、TAB3使TAK1与K63连接的多聚泛素链上的TRAF6结合,触发p38α和JNK的MAPK途径中TAK1的激活 Following TLR or IL-1R ligation, MyD88 is recruited to the TIR domain of the receptor. For TLR2 and TLR4, this recruitment requires MyD88 adaptor-like protein (MAL, also known as TIRAP). MyD88 and the serine/threonine kinase IL-1 receptor-associated kinase 4 (IRAK4) interact via their death domains. IRAK4 catalytic activity subsequently induces a complex with IRAK1 and IRAK2 and the E3 ubiquitin ligase TRAF6. UBC13, together with either TRAF6 or an unidentified E3 ligase, catalyzes the formation of K63-linked polyubiquitin chains on TRAF6 and IRAK1.TAK1-binding protein 2 (TAB2) and TAB3 recruit TGFβ-activated kinase 1 (TAK1) to K63-linked polyubiquitylated TRAF6, which triggers TAK1 activation of the p38α and JNK MAPK pathways. |
|
|
MAP2K中MAPK对接域的存在确保MAP2Ks对MAPK磷酸化的特异性,而MAPK对接域同样对下游MAPK具有特异性。MAP2K本身由MAPK激酶(MAP3Ks)对其激活环的丝氨酸和/或苏氨酸磷酸化激活[31]。MAP3Ks通过细胞自主或非细胞自主机制激活ERK通路,有助于发现RAS-RAF驱动的肿瘤信号传导通路以外的癌症进展[30]。有文献报道,MAP3K在不同癌症中发挥不同作用,MAP3K1的表达在胰腺导管腺癌中具有促肿瘤作用,MAP3K3则显示抗肿瘤作用,而MAP3K2显示无相关性,这些研究进一步突出MAP3Ks在疾病进展中的重要性[37]。
MAP3Ks激活过程相当复杂,不同MAP3Ks能以多种形式激活,包括上游激酶的磷酸化或与信号转接器和小GTP酶的相互作用。转化生长因子β活化激酶1 (transforming growth facter-β activated kinase 1)作为p38和JNK通路的直接激活MAP3K,也间接促进TPL2激活[30]。TPL2则通过IκB激酶(inhibitor of kappa B kinase,IKK)诱导NF-κB亚基前体蛋白p105的水解激活ERK1/2[38]。支架蛋白可以将MAPK级联的不同层连接成多酶复合物,这保证了信号的特异性,也调节了信号的振幅和持续时间[36]。这类适配蛋白也可能对先天免疫反应中的MAPK激活起重要作用,但这是一个相对未开发的领域[30-31]。
4 MAPK抗菌作用巨噬细胞产生活性氧(reactive oxygen species,ROS)、一氧化氮(nitric oxide,NO)和吞噬作用直接杀死病原体。此外,它们产生细胞因子和趋化因子能够促进其他免疫细胞的招募和激活,从而诱导炎症和适应性免疫反应。MAPK信号通路对一氧化氮合酶(inducible nitric oxide synthase,iNOS)的调控一直存在争议[39]。一些研究显示,ERK1/2通路抑制剂对iNOS表达无影响,而iNOS使用精氨酸作为底物,ERK1/2和p38α又被证实可以调节脂多糖(lipopolysaccharide,LPS)诱导的巨噬细胞对精氨酸的摄取[40]。
ROS由多亚基NADPH氧化酶复合体产生,该复合体组装过程依赖p47phox亚基的磷酸化。在巨噬细胞中,p38介导p47phox磷酸化依赖于大肠杆菌或肿瘤坏死因子刺激,ERK1/2则在粒细胞/巨噬细胞集落刺激因子的下游发挥作用[41]。
吞噬作用同样受到MAPK信号通路调节。抑制ERK1/2激活可以减弱对弗朗西斯菌的吞噬作用,而阻断p38α则可以减弱对大肠杆菌的吞噬作用并影响自身吞噬体的成熟[42]。
5 病原操纵MAPK途径促进自身感染机制许多病原体已经进化出抑制免疫反应的机制,这往往与其毒性增加有关。一种常见的抑制策略是针对宿主细胞内的信号网络,包括那些调节NF-κB和IRF转录因子的信号网络[43-44]。考虑到MAPK信号通路在调节免疫反应中的重要性,一些病原体能够进化出直接调节MAPK激活的机制也不足为奇。
MAPKs抗菌功能主要表现在不同级联激酶磷酸化过程,除Thr-X-Tyr基序外,MAP3Ks序列中还存在其他基序,能够选择性赋予下游对不同刺激的活性。在长期进化过程中,病原也逐渐进化出精确调节这些基序的能力。病原调控MAPK途径方式主要分成3类。
5.1 表达降解MAPK级联特定成分蛋白酶GP63被称为主要表面蛋白酶(major surface protease,MSP)或利什曼溶血素(leishmanolysin),是覆盖在利什曼原虫(Leishmania,L. major)前鞭毛体上含量最丰富的蛋白[45]。一方面,不同寄生虫模型研究表明,利什曼原虫在周围环境中释放GP63能促进自身繁殖;另一方面,经GP63处理的纤维连接蛋白片段通过减少活性氧中间体的产生起到保护巨噬细胞内的寄生虫的作用[45-46]。在利什曼原虫感染对成纤维细胞信号传导的研究中发现,L. major以GP63依赖方式下调p38,这种下调方式与GP63介导的转化生长因子激活激酶结合蛋白1 (TAK-1-binding protein-1,TAB1)水解保持一致[46-47]。
5.2 抑制MAPK级联激活病原抑制MAPK级联激活机制大致划分为两类,一类是靶向MAP2K破坏其激酶蛋白活性实现;另一类病原利用蛋白翻译后修饰影响MAPK磷酸化抑制级联激活[48]。
炭疽杆菌能够分泌2种重要毒力因子:致死因子(lethal factor,LF)和水肿因子(edema factor,EF)[49]。这2种毒素经保护性抗原(protective antigen,PA)转运至细胞,其中Zn2+依赖性金属蛋白酶LF切割MKKs的N端,从而削弱其功能[50]。MAPK通路在所有细胞类型中起着增殖、生存和炎症等重要作用,而MAPK通路的破坏会导致宿主的多系统功能障碍。巨噬细胞和树突状细胞中MAPK通路失活导致促炎细胞因子分泌受到抑制,共刺激分子如CD80和CD86下调,并导致T细胞启动无效,最终结果是先天和适应性免疫反应受损[51]。血管系统的内皮细胞在LT暴露后发生凋亡,也可能与MAPK通路失活有关;而EF是一种腺苷酸环化酶,由钙调素通过非常规方式激活,与LF一样,EF主要作用是通过削弱吞噬细胞功能破坏宿主防御[49]。中性粒细胞趋化、吞噬、超氧化物产生和杀菌活性,这些效应一般归因于蛋白激酶A (protein kinase A,PKA)的激活,正常生理中PKA活动受到严格调控,当炭疽杆菌感染后,EF抑制TNF-α产生同时诱导IL-6水平升高,这个过程中cAMP的升高能够阻断由于LPS引起的ERK、JNK通路的活化[52]。
与炭疽杆菌不同,耶尔森氏菌(Yersinia spp.)、沙门氏菌(Salmonella)、副溶血弧菌(Vibrio parahemolyticus)等病原菌通过介导MAPK乙酰化从而实现信号阻断[53]。III型分泌系统(type III secretion systems,TTSSs)作为革兰氏阴性菌中特异性蛋白输出途径,能够协助病原感染动物和植物,并且TTSSs往宿主细胞注入效应蛋白耶尔森菌外蛋白(Yersinia outer proteins,Yops)能够抵消刺激带来的先天和适应性免疫反应,其中耶尔森菌外蛋白J (Yersinia outer protein J,YopJ)和耶尔森菌外蛋白P (Yersinia outer protein P,YopP)作为研究的热点,更是与多条信号通路相关联[54-55]。
目前,已经证实YopJ诱导细胞凋亡和抑制细胞因子产生是其失活或抑制MAPK和NF-κB信号通路的结果[56]。有文献报道证实,YopJ与MKKs家族成员结合,并阻断其被磷酸化激活[57]。根据预测,YopJ与CE家族的半胱氨酸蛋白酶具有相似二级结构,其中包括泛素样蛋白酶家族(ubiquitin-like protease,UBLs)。这些酶含有催化所需的3种残基(His、Asp/Glu和Cys),YopJ生物学功能同样需要这些残基。YopJ活性与Ub样蛋白小Ub相关修饰蛋白(small Ub-related modifier,SUMO)整体修饰水平相关。由此提出,YopJ从信号蛋白中去除Ub样修饰,从而抑制MAPK和NF-κB反应途径;后续研究发现,YopJ通过蛋白互作方式与MKKs或IKK结合,并使用乙酰辅酶A作为辅助因子,使激活环中的丝氨酸和苏氨酸残基乙酰化,从而阻止上游TAK1的激活信号[58]。类似的乙酰化过程在鼠伤寒沙门氏菌中则是由AvrA效应蛋白靶向JNK MAPK调节哺乳动物肠道免疫和生存反应。
5.3 增强MAPK级联激活除上述2种调节方式外,病原体编码的蛋白也可能通过激活而不是抑制MAPK活性的方式促进自身感染。
细胞内细菌的生长速率被促进复制和抑制复制的毒力蛋白严密控制。肠道链球菌进入宿主细胞后会在膜结合区室中复制,这一过程被周围纤维状肌动蛋白网的形成所抑制,纤维状肌动蛋白网的形成是由肠道链球菌效应分子SteC触发的[59]。SteC作为一种丝氨酸/苏氨酸激酶,与C-Raf激酶结构域具有相似性,推测能够模仿C-Raf功能直接磷酸化MEK;此外,SteC刺激MAPK过程检测到S200介导的MEK1自磷酸化,S200磷酸化会取代已知MEK活化残基S218和S222上引起自磷酸化的负调节螺旋[60]。
有文献报道,肠道链球菌效应蛋白SpvC通过自身磷酸苏氨酸裂解酶活性灭活ERK1/2,这也反映了病原会根据感染周期的不同阶段、不同程度选择性调节MAPK活性的能力[59]。在感染前期,链球菌依赖SteC激活ERK1/2使得IL-10增加、促炎反应减弱,有助于细菌感染巨噬细胞并建立稳定的感染;感染建立后,SpvC水平升高使得ERK1/2失活,便于细菌进行下一步感染[61]。
这类现象在病毒中也有所报道,如丙型肝炎病毒(hepatitis C virus,HCV)感染过程中触发TAB1和p38相互作用,诱导p38自磷酸化,活化的p38反过来催化HCV核心蛋白磷酸化,实现核心齐聚和病毒组装[62]。这种前馈循环使得HCV感染变成恶性循环[63]。从理论上来说,打破这种恶性循环对于抵抗病原感染是有效的,因此,开发靶向MAPK途径的药物对于疾病的治疗具有积极意义,另一方面也反映了揭示病原感染MAPK途径具体机制对于新型药物开发的极大参考价值。
6 总结与展望回顾以前关于细菌病原体调节MAPK信号传导的研究,发现大多数病原体已经发展出直接抑制MAPK激活以满足自身感染的机制。然而,一个例外是病原体鼠伤寒沙门氏菌血清型(鼠伤寒沙门氏菌),它使用III型分泌效应器SteC通过激活MEK和ERK的信号通路来促进肌动蛋白细胞骨架重组,从而有助于控制该病原体的细胞内复制[13, 60]。沙门氏菌的细胞内生长速率是一个严格控制的过程,涉及复制促进和复制抑制毒力蛋白活性之间的平衡,因此,在感染期间抑制细菌增殖对于沙门氏菌非常重要。
另外,单增李斯特菌在感染不同宿主和细胞时可以选择性激活或抑制MAPK途径信号调控自身感染,由此猜测单增李斯特菌已经进化出复杂的机制,可以在各种感染条件下灵活地调节宿主细胞的信号传导实现感染[64]。有研究发现,Caco-2细胞中的MAPK ERK1/2磷酸化是由单核细胞增生李斯特菌感染强烈触发的,此过程高度依赖于李斯特菌溶血素O的膜透化活性;LLO对ERK1/2磷酸化的激活作用是浓度依赖性的,并且5 nmol/L浓度足以触发这种细胞反应;胆固醇可以完全抑制该过程,从而阻断LLO的膜穿孔能力[14]。先前的研究也表明,炎症小体在感染过程中被李斯特菌激活,一些研究人员认为caspase-1激活是LLO依赖性的,炎症小体的这种激活是由LLO在质膜上的孔形成活性介导的[65-66]。尽管LLO在单增李斯特菌感染期间调节MAPK信号传导的机制仍然难以捉摸,但基于这些研究,推测LLO的成孔活性在连接LLO介导的MAPK信号传导和宿主免疫调节中起主要作用,并且这可能与MAPK磷酸化有密切关联。
信号通路磷酸化途径改变会导致严重疾病,许多信号传导途径级联反应磷酸化-去磷酸化失调被公认为是癌症发生的前提。然而应激激活的MAPK途径,如JNK和p38对于细胞周期过程、酪氨酸激酶的激活过程起着重要的调节作用,可以改变癌细胞对靶向治疗和化疗的反应[67]。
为治疗MAPK途径异常导致的疾病,一方面可以通过对应通路抑制剂实现[68]。有研究表明,靶向MAPK途径是治疗某些先天免疫疾病的可行目标,而且最突出的临床应用靶点是ERK途径[69]。选择ERK抑制剂与上游抑制的联合使用可能会产生更理想的治疗效果,根据肿瘤类型和状态,同一靶点的不同抑制剂或同一途径中不同靶点的药物组合,与单一抑制剂相比可导致显著的疗效差异,因此,靶向磷酸化途径的药物研发成为癌症治疗的聚焦研究领域,从而使得围绕MAPK途径开展的研究显得尤为重要[70]。另一方面,病原调控MAPK途径也为减毒活疫苗开发与应用提供借鉴。例如,单增李斯特菌作为一种优质疫苗载体得到了广泛的应用。目前,已经有关于李斯特菌狗骨肉瘤疫苗在临床上的应用,启示我们可以在某些疫苗载体基础上开发针对特定疾病的新式药物[71-72]。通过对病原激活MAPK途径具体机制的深入了解,开发出能够靶向MAPK关键激酶蛋白的减毒活疫苗,从而实现疾病治疗的方案也将作为后续研究的重点。
| [1] |
Takeuchi O, Akira S. Pattern recognition receptors and inflammation[J]. Cell, 2010, 140(6): 805-820. DOI:10.1016/j.cell.2010.01.022 |
| [2] |
Rumpret M, Von Richthofen HJ, Peperzak V, Meyaard L. Inhibitory pattern recognition receptors[J]. The Journal of Experimental Medicine, 2022, 219(1): e20211463. DOI:10.1084/jem.20211463 |
| [3] |
Zepeda-Cervantes J, Ramírez-Jarquín JO, Vaca L. Interaction between virus-like particles (VLPs) and pattern recognition receptors (PRRs) from dendritic cells (DCs): toward better engineering of VLPs[J]. Frontiers in Immunology, 2020, 11: 1100. DOI:10.3389/fimmu.2020.01100 |
| [4] |
Liu CH, Liu HY, Ge BX. Innate immunity in tuberculosis: host defense vs. pathogen evasion[J]. Cellular & Molecular Immunology, 2017, 14(12): 963-975. |
| [5] |
Ohto U. Activation and regulation mechanisms of NOD-like receptors based on structural biology[J]. Frontiers in Immunology, 2022, 13: 953530. DOI:10.3389/fimmu.2022.953530 |
| [6] |
Liu J, Zhang H, Su YH, Zhang BJ. Application and prospect of targeting innate immune sensors in the treatment of autoimmune diseases[J]. Cell & Bioscience, 2022, 12(1): 68. |
| [7] |
Grave N, Scheffel TB, Cruz FF, Rockenbach L, Goettert MI, Laufer S, Morrone FB. The functional role of p38 MAPK pathway in malignant brain tumors[J]. Frontiers in Pharmacology, 2022, 13: 975197. DOI:10.3389/fphar.2022.975197 |
| [8] |
Farkhondeh T, Mehrpour O, Buhrmann C, Pourbagher-Shahri AM, Shakibaei M, Samarghandian S. Organophosphorus compounds and MAPK signaling pathways[J]. International Journal of Molecular Sciences, 2020, 21(12): E4258. DOI:10.3390/ijms21124258 |
| [9] |
Fiil BK, Petersen K, Petersen M, Mundy J. Gene regulation by MAP kinase cascades[J]. Current Opinion in Plant Biology, 2009, 12(5): 615-621. DOI:10.1016/j.pbi.2009.07.017 |
| [10] |
Zhao HK, Wu L, Yan GF, Chen Y, Zhou MY, Wu YZ, Li YS. Inflammation and tumor progression: signaling pathways and targeted intervention[J]. Signal Transduction and Targeted Therapy, 2021, 6: 263. DOI:10.1038/s41392-021-00658-5 |
| [11] |
Chen Y, Yang J, Huang Z, Yin B, Umar T, Yang C, Zhang X, Jing H, Guo S, Guo M, et al. Vitexin mitigates Staphylococcus aureus-induced mastitis via regulation of ROS/ER stress/NF-κB/MAPK pathway[J]. Oxidative Medicine and Cellular Longevity, 2022, 7977433. DOI:10.1155/2022/7977433 |
| [12] |
Cui L, Shao X, Sun W, Zheng F, Dong J, Li J, Wang H, Li J. Anti-inflammatory effects of progesterone through NF-κB and MAPK pathway in lipopolysaccharide- or Escherichia coli-stimulated bovine endometrial stromal cells[J]. PLoS One, 2022, 17(4): e0266144. DOI:10.1371/journal.pone.0266144 |
| [13] |
Zhang L, Sun Y, Xu W, Geng Y, Su YH, Wang QN, Wang JL. Baicalin inhibits Salmonella typhimurium-induced inflammation and mediates autophagy through TLR4/MAPK/NF-κB signalling pathway[J]. Basic & Clinical Pharmacology & Toxicology, 2021, 128(2): 241-255. |
| [14] |
Cheng CY, Sun J, Yu HF, Ma TT, Guan CY, Zeng H, Zhang X, Chen ZW, Song HH. Listeriolysin O pore-forming activity is required for ERK1/2 phosphorylation during Listeria monocytogenes infection[J]. Frontiers in Immunology, 2020, 11: 1146. DOI:10.3389/fimmu.2020.01146 |
| [15] |
Nguyen BN, Peterson BN, Portnoy DA. Listeriolysin O: a phagosome-specific cytolysin revisited[J]. Cellular Microbiology, 2019, 21(3): e12988. DOI:10.1111/cmi.12988 |
| [16] |
Osborne SE, Brumell JH. Listeriolysin O: from bazooka to Swiss army knife[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2017, 372(1726): 20160222. DOI:10.1098/rstb.2016.0222 |
| [17] |
Hamerman JA, Pottle J, Ni MJ, He YT, Zhang ZY, Buckner JH. Negative regulation of TLR signaling in myeloid cells—implications for autoimmune diseases[J]. Immunological Reviews, 2016, 269(1): 212-227. DOI:10.1111/imr.12381 |
| [18] |
Simpson DS, Pang JY, Weir A, Kong IY, Fritsch M, Rashidi M, Cooney JP, Davidson KC, Speir M, Djajawi TM, et al. Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway[J]. Immunity, 2022, 55(3): 423-441.e9. DOI:10.1016/j.immuni.2022.01.003 |
| [19] |
Luchner M, Reinke S, Milicic A. TLR agonists as vaccine adjuvants targeting cancer and infectious diseases[J]. Pharmaceutics, 2021, 13(2): 142. DOI:10.3390/pharmaceutics13020142 |
| [20] |
Kirtland ME, Tsitoura DC, Durham SR, Shamji MH. Toll-like receptor agonists as adjuvants for allergen immunotherapy[J]. Frontiers in Immunology, 2020, 11: 599083. DOI:10.3389/fimmu.2020.599083 |
| [21] |
Peng J, Yuan Q, Lin B, Panneerselvam P, Wang X, Luan XL, Lim SK, Leung BP, Ho B, Ding JL. SARM inhibits both TRIF- and MyD88-mediated AP-1 activation[J]. European Journal of Immunology, 2010, 40(6): 1738-1747. DOI:10.1002/eji.200940034 |
| [22] |
Qian J, Xu HD, Lv DQ, Liu W, Chen EG, Zhou Y, Wang Y, Ying KJ, Fan XH. Babaodan controls excessive immune responses and may represent a cytokine-targeted agent suitable for COVID-19 treatment[J]. Biomedicine & Pharmacotherapy, 2021, 139: 111586. |
| [23] |
Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling[J]. Nature, 2010, 465(7300): 885-890. DOI:10.1038/nature09121 |
| [24] |
Hu YH, Wang Y, Wang F, Dong YM, Jiang WL, Wang YP, Zhong X, Ma LX. SPOP negatively regulates toll-like receptor-induced inflammation by disrupting MyD88 self-association[J]. Cellular & Molecular Immunology, 2021, 18(7): 1708-1717. |
| [25] |
Mulas F, Wang X, Song SS, Nishanth G, Yi WJ, Brunn A, Larsen PK, Isermann B, Kalinke U, Barragan A, et al. The deubiquitinase OTUB1 augments NF-κB-dependent immune responses in dendritic cells in infection and inflammation by stabilizing UBC13[J]. Cellular & Molecular Immunology, 2021, 18(6): 1512-1527. |
| [26] |
Zhao W, Ma L, Cai C, Gong X. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages[J]. International Journal of Biological Sciences, 2019, 15(8): 1571-1581. DOI:10.7150/ijbs.34211 |
| [27] |
An YN, Zhang HF, Wang C, Jiao FT, Xu HY, Wang XF, Luan WJ, Ma FX, Ni LH, Tang XD, et al. Activation of ROS/MAPK s/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis[J]. The FASEB Journal, 2019, 33(11): 12515-12527. DOI:10.1096/fj.201802805RR |
| [28] |
Qu C, Park JY, Yun MW, He QT, Yang F, Kim K, Ham D, Li RR, Iverson TM, Gurevich VV, et al. Scaffolding mechanism of arrestin-2 in the cRaf/MEK1/ERK signaling cascade[J]. PNAS, 2021, 118(37): e2026491118. DOI:10.1073/pnas.2026491118 |
| [29] |
Aikin TJ, Peterson AF, Pokrass MJ, Clark HR, Regot S. MAPK activity dynamics regulate non-cell autonomous effects of oncogene expression[J]. eLife, 2020, 9: e60541. DOI:10.7554/eLife.60541 |
| [30] |
Yue JC, López JM. Understanding MAPK signaling pathways in apoptosis[J]. International Journal of Molecular Sciences, 2020, 21(7): 2346. DOI:10.3390/ijms21072346 |
| [31] |
Hutton SR, Otis JM, Kim EM, Lamsal Y, Stuber GD, Snider WD. ERK/MAPK signaling is required for pathway-specific striatal motor functions[J]. The Journal of Neuroscience, 2017, 37(34): 8102-8115. DOI:10.1523/JNEUROSCI.0473-17.2017 |
| [32] |
Bengal E, Aviram S, Hayek T. p38 MAPK in glucose metabolism of skeletal muscle: beneficial or harmful?[J]. International Journal of Molecular Sciences, 2020, 21(18): 6480. DOI:10.3390/ijms21186480 |
| [33] |
Cai JY, Gao L, Wang YX, Li Y, Ye Z, Tong S, Yan TF, sun Q, Xu Y, Jiang HX, et al. TMBIM1 promotes proliferation and attenuates apoptosis in glioblastoma cells by targeting the p38 MAPK signalling pathway[J]. Translational Oncology, 2022, 19: 101391. DOI:10.1016/j.tranon.2022.101391 |
| [34] |
Xu Y, Sun Q, Yuan FE, Dong HM, Zhang HK, Geng RX, Qi YZ, Xiong XX, Chen QX, Liu BH. RND2 attenuates apoptosis and autophagy in glioblastoma cells by targeting the p38 MAPK signalling pathway[J]. Journal of Experimental & Clinical Cancer Research: CR, 2020, 39(1): 174. |
| [35] |
Knight JRP, Alexandrou C, Skalka GL, Vlahov N, Pennel K, Officer L, Teodosio A, Kanellos G, Gay DM, May-Wilson S, et al. MNK inhibition sensitizes KRAS-mutant colorectal cancer to mTORC1 inhibition by reducing eIF4E phosphorylation and c-MYC expression[J]. Cancer Discovery, 2021, 11(5): 1228-1247. DOI:10.1158/2159-8290.CD-20-0652 |
| [36] |
Chen J, Wang LH, Yuan M. Update on the roles of rice MAPK cascades[J]. International Journal of Molecular Sciences, 2021, 22(4): 1679. DOI:10.3390/ijms22041679 |
| [37] |
Nguyen K, Tran MN, Rivera A, Cheng T, Windsor GO, Chabot AB, Cavanaugh JE, Collins-Burow BM, Lee SB, Drewry DH, et al. MAP3K family review and correlations with patient survival outcomes in various cancer types[J]. Frontiers in Bioscience: Landmark Edition, 2022, 27(5): 167. DOI:10.31083/j.fbl2705167 |
| [38] |
Nakano R, Kitanaka T, Namba S, Kitanaka N, Suwabe Y, Konno T, Yamazaki J, Nakayama T, Sugiya H. Non-transcriptional and translational function of canonical NF-κB signaling in activating ERK1/2 in IL-1β-induced COX-2 expression in synovial fibroblasts[J]. Frontiers in Immunology, 2020, 11: 579266. DOI:10.3389/fimmu.2020.579266 |
| [39] |
Ahmad N, Ansari MY, Bano S, Haqqi TM. Imperatorin suppresses IL-1β-induced iNOS expression via inhibiting ERK-MAPK/AP1 signaling in primary human OA chondrocytes[J]. International Immunopharmacology, 2020, 85: 106612. DOI:10.1016/j.intimp.2020.106612 |
| [40] |
Yang ZJ, Wang SC, Liu HG, Xu SW. MAPK/iNOS pathway is involved in swine kidney necrosis caused by cadmium exposure[J]. Environmental Pollution, 2021, 274: 116497. DOI:10.1016/j.envpol.2021.116497 |
| [41] |
Kumar RR, Arora K, Goswami S, Sakhare A, Singh B, Chinnusamy V, Praveen S. MAPK enzymes: a ROS activated signaling sensors involved in modulating heat stress response, tolerance and grain stability of wheat under heat stress[J]. 3 Biotech, 2020, 10(9): 380. DOI:10.1007/s13205-020-02377-0 |
| [42] |
Tóthová Z, Šemeláková M, Solárová Z, Tomc J, Debeljak N, Solár P. The role of PI3K/AKT and MAPK signaling pathways in erythropoietin signalization[J]. International Journal of Molecular Sciences, 2021, 22(14): 7682. DOI:10.3390/ijms22147682 |
| [43] |
Ivashkiv LB. Inflammatory signaling in macrophages: transitions from acute to tolerant and alternative activation states[J]. European Journal of Immunology, 2011, 41(9): 2477-2481. DOI:10.1002/eji.201141783 |
| [44] |
Heinz LX, Lee J, Kapoor U, Kartnig F, Sedlyarov V, Papakostas K, César-Razquin A, Essletzbichler P, Goldmann U, Stefanovic A, et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7–9[J]. Nature, 2020, 581(7808): 316-322. DOI:10.1038/s41586-020-2282-0 |
| [45] |
Martinez PA, Petersen CA. Chronic infection by Leishmania amazonensis mediated through MAPK ERK mechanisms[J]. Immunologic Research, 2014, 59(1/2/3): 153-165. |
| [46] |
Hallé M, Gomez MA, Stuible M, Shimizu H, McMaster WR, Olivier M, Tremblay ML. The Leishmania surface protease GP63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation[J]. Journal of Biological Chemistry, 2009, 284(11): 6893-6908. DOI:10.1074/jbc.M805861200 |
| [47] |
Valentim-Silva JR, Macedo SRA, De Barros NB, Dos Santos Ferreira A, Da Silva JHM, De Figueiredo Nicolete LD, Nicolete R. Antileishmanial drugs activate inflammatory signaling pathways via toll-like receptors (docking approach) from Leishmania amazonensis-infected macrophages[J]. Int Immunopharmacol, 2020, 85: 106640. DOI:10.1016/j.intimp.2020.106640 |
| [48] |
Braicu C, Buse M, Busuioc C, Drula R, Gulei DA, Raduly L, Rusu A, Irimie A, Atanasov AG, Slaby O, et al. A comprehensive review on MAPK: a promising therapeutic target in cancer[J]. Cancers, 2019, 11(10): 1618. DOI:10.3390/cancers11101618 |
| [49] |
Yang NJ, Isensee J, Neel DV, Quadros AU, Zhang HXB, Lauzadis J, Liu SM, Shiers S, Belu A, Palan S, et al. Anthrax toxins regulate pain signaling and can deliver molecular cargoes into ANTXR2+ DRG sensory neurons[J]. Nature Neuroscience, 2022, 25(2): 168-179. DOI:10.1038/s41593-021-00973-8 |
| [50] |
Xie T, Auth RD, Frucht DM. The effects of anthrax lethal toxin on host barrier function[J]. Toxins, 2011, 3(6): 591-607. DOI:10.3390/toxins3060591 |
| [51] |
Zhang X, Feng T, Zhou X, Sullivan PM, Hu F, Lou Y, Yu J, Feng J, Liu H, Chen Y. Inactivation of TMEM106A promotes lipopolysaccharide-induced inflammation via the MAPK and NF-κB signaling pathways in macrophages[J]. Clinical and Experimental Immunology, 2020, 203(1): 125-136. DOI:10.1111/cei.13528 |
| [52] |
Raymond B, Ravaux L, Mémet S, Wu YZ, Sturny-Leclère A, Leduc D, Denoyelle C, Goossens PL, Payá M, Raymondjean M, et al. Anthrax lethal toxin down-regulates type-IIA secreted phospholipase A2 expression through MAPK/NF-κB inactivation[J]. Biochemical Pharmacology, 2010, 79(8): 1149-1155. DOI:10.1016/j.bcp.2009.11.023 |
| [53] |
Haase R, Richter K, Pfaffinger G, Courtois G, Ruckdeschel K. Yersinia outer protein P suppresses TGF-beta-activated kinase-1 activity to impair innate immune signaling in Yersinia enterocolitica-infected cells[J]. Journal of Immunology: Baltimore, Md: 1950, 2005, 175(12): 8209-8217. |
| [54] |
Sawa T, Katoh H, Yasumoto H. V-antigen homologs in pathogenic gram-negative bacteria[J]. Microbiology and Immunology, 2014, 58(5): 267-285. DOI:10.1111/1348-0421.12147 |
| [55] |
Harmon DE, Davis AJ, Castillo C, Mecsas J. Identification and characterization of small-molecule inhibitors of yop translocation in Yersinia pseudotuberculosis[J]. Antimicrobial Agents and Chemotherapy, 2010, 54(8): 3241-3254. DOI:10.1128/AAC.00364-10 |
| [56] |
Zhang Y, Ting AT, Marcu KB, Bliska JB. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia[J]. Journal of Immunology: Baltimore, Md: 1950, 2005, 174(12): 7939-7949. |
| [57] |
Sheppe AEF, Santelices J, Czyz DM, Edelmann MJ. Yersinia pseudotuberculosis YopJ limits macrophage response by downregulating COX-2-mediated biosynthesis of PGE2 in a MAPK/ERK-dependent manner[J]. Microbiology Spectrum, 2021, 9(1): e0049621. DOI:10.1128/Spectrum.00496-21 |
| [58] |
Wiley DJ, Shrestha N, Yang J, Atis N, Dayton K, Schesser K. The activities of the Yersinia protein kinase A (YpkA) and outer protein J (YopJ) virulence factors converge on an eIF2α kinase[J]. Journal of Biological Chemistry, 2009, 284(37): 24744-24753. DOI:10.1074/jbc.M109.010140 |
| [59] |
Wang Q, Zhou H, Fan H, Wang X. Coinfection with porcine circovirus type 2 (PCV2) and Streptococcus suis serotype 2 (SS2) enhances the survival of SS2 in swine tracheal epithelial cells by decreasing reactive oxygen species production[J]. Infection and Immunity, 2020, 88(11): e00537-e00520. |
| [60] |
Odendall C, Rolhion N, Förster A, Poh J, Lamont DJ, Liu M, Freemont PS, Catling AD, Holden DW. The Salmonella kinase SteC targets the MAP kinase MEK to regulate the host actin cytoskeleton[J]. Cell Host & Microbe, 2012, 12(5): 657-668. |
| [61] |
Mazurkiewicz P, Thomas J, Thompson JA, Liu M, Arbibe L, Sansonetti P, Holden DW. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases[J]. Molecular Microbiology, 2008, 67(6): 1371-1383. DOI:10.1111/j.1365-2958.2008.06134.x |
| [62] |
Cheng YT, Sun F, Wang LY, Gao MJ, Xie YL, Sun Y, Liu H, Yuan YF, Yi W, Huang Z, et al. Virus-induced p38 MAPK activation facilitates viral infection[J]. Theranostics, 2020, 10(26): 12223-12240. DOI:10.7150/thno.50992 |
| [63] |
Song X, Gao X, Wang Y, Raja R, Zhang Y, Yang S, Li M, Yao Z, Wei L. HCV core protein induces chemokine CCL2 and CXCL10 expression through NF-κB signaling pathway in macrophages[J]. Frontiers in Immunology, 2021, 12: 654998. DOI:10.3389/fimmu.2021.654998 |
| [64] |
Hashino M, Tachibana M, Nishida T, Hara H, Tsuchiya K, Mitsuyama M, Watanabe K, Shimizu T, Watarai M. Inactivation of the MAPK signaling pathway by Listeria monocytogenes infection promotes trophoblast giant cell death[J]. Frontiers in Microbiology, 2015, 6: 1145. |
| [65] |
Hara H, Tsuchiya K, Nomura T, Kawamura I, Shoma S, Mitsuyama M. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm[J]. Journal of Immunology: Baltimore, Md: 1950, 2008, 180(12): 7859-7868. |
| [66] |
Megli C, Morosky S, Rajasundaram D, Coyne CB. Inflammasome signaling in human placental trophoblasts regulates immune defense against Listeria monocytogenes infection[J]. The Journal of Experimental Medicine, 2021, 218(1): e20200649. DOI:10.1084/jem.20200649 |
| [67] |
Arafa ESA, Refaey MS, Abd El-Ghafar OAM, Hassanein EHM, Sayed AM. The promising therapeutic potentials of ginsenosides mediated through p38 MAPK signaling inhibition[J]. Heliyon, 2021, 7(11): e08354. DOI:10.1016/j.heliyon.2021.e08354 |
| [68] |
Czarnecka AM, Bartnik E, Fiedorowicz M, Rutkowski P. Targeted therapy in melanoma and mechanisms of resistance[J]. International Journal of Molecular Sciences, 2020, 21(13): 4576. DOI:10.3390/ijms21134576 |
| [69] |
Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, Carr D, Deng Y, Jin W, Black S, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors[J]. Cancer Discovery, 2013, 3(7): 742-750. DOI:10.1158/2159-8290.CD-13-0070 |
| [70] |
Ullah R, Yin Q, Snell AH, Wan LX. RAF-MEK-ERK pathway in cancer evolution and treatment[J]. Seminars in Cancer Biology, 2022, 85: 123-154. DOI:10.1016/j.semcancer.2021.05.010 |
| [71] |
Cheng CY, Jiang L, Ma TT, Wang H, Han X, Sun J, Yang YC, Chen ZW, Yu HF, Hang Y, et al. Carboxyl-terminal residues N478 and V479 required for the cytolytic activity of listeriolysin O play a critical role in Listeria monocytogenes pathogenicity[J]. Frontiers in Immunology, 2017, 8: 1439. DOI:10.3389/fimmu.2017.01439 |
| [72] |
Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, Wallecha A, Huebner M, Paterson Y. Immunotherapy with a HER2-targeting Listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma[J]. Clinical Cancer Research, 2016, 22(17): 4380-4390. DOI:10.1158/1078-0432.CCR-16-0088 |
 2022, Vol. 49
2022, Vol. 49




